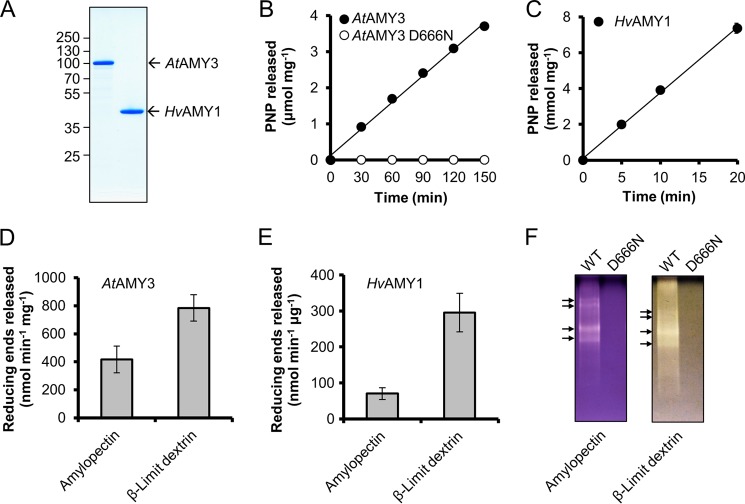
FIGURE 1.
Asp666 is essential for the amylolytic activity of AtAMY3. A, SDS-PAGE. Purified recombinant AtAMY3 (1 μg) and HvAMY1 (1 μg) were analyzed by SDS-PAGE in a 10% (w/v) gel stained with Coomassie Brilliant Blue. B and C, activity against BPNP-G7 of AtAMY3 WT and AtAMY3 D666N at pH 7.9 (B) and HvAMY1 at pH 5.5 (C). The purified proteins (10 μg of AtAMY3s and 10 ng of HvAMY1) were incubated for the indicated times with an excess of BPNP-G7 and α-glucosidase at 37 °C. Error bars indicate mean ± S.E. (n = 3) and are mostly smaller than the symbols. D, activity of AtAMY3 against amylopectin and β-limit dextrin. Recombinant protein (1 μg) was incubated for 30 min with 1.5 mg of solubilized substrate, and the amount of reducing ends released was assayed. Values represent the mean ± S.E. (n = 4). E, activity of HvAMY1 against amylopectin and β-limit dextrin. Recombinant protein (10 ng) was incubated for 10 min with 1.5 mg of solubilized substrate, and the amount of reducing ends released was assayed. Values represent the mean ± S.E. (n = 3). F, activity of AtAMY3 WT and D666N on native PAGE containing 7.5% acrylamide and 0.1% amylopectin or β-limit dextrin. Recombinant proteins (1 μg) were separated by native-PAGE for 3 h at 4 °C. Amylolytic activities were detected by staining with iodine solution after incubation for 1 h. Distinct AtAMY3 activities are indicated with arrows. One representative gel from five replicate gels is shown.