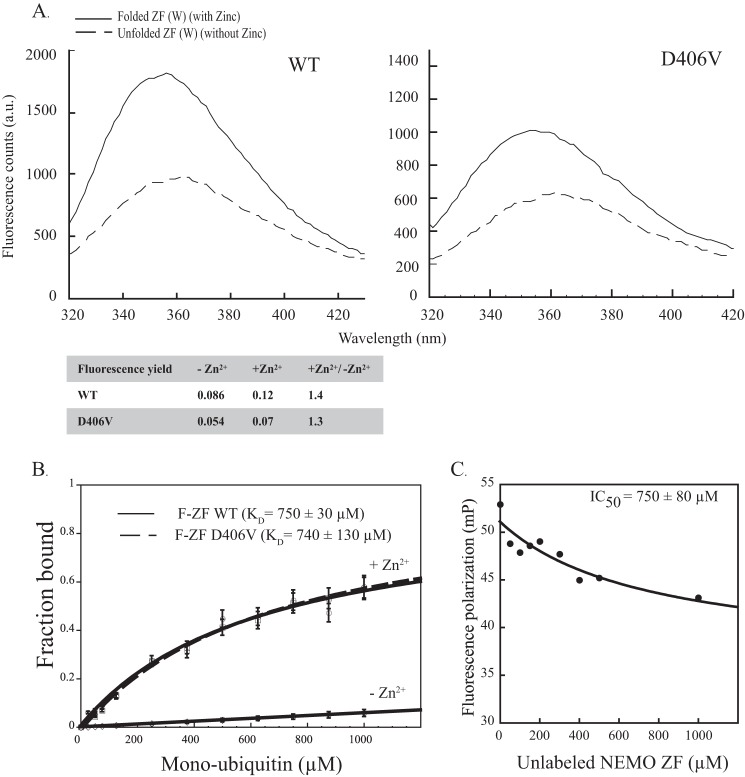
FIGURE 2.
Neither the zinc-induced folding of NEMO ZF nor its mono-ubiquitin binding property are affected by the D406V mutation. A, the emission spectra (305–450 nm) of ZF (W) mutant peptides. WT and D406V mutant, subtracted from buffer signal were recorded upon tryptophan excitation at 295 nm. ZF (W) peptides at 25 μm were tested in the absence or presence of zinc. The addition of zinc led to a similar fluorescence yield for the two peptides WT and D406V, as indicated in the table below. a.u., absorbance units. B, affinities of fluorescein-labeled NEMO ZF (F-ZF) and its D406V mutant for mono-ubiquitin measured by fluorescence polarization. The dissociation constants (KD) were determined using ZF concentrations of 0.1 μm and increasing concentrations of mono-ubiquitin as described under “Experimental Procedures.” No significant binding was observed between unfolded ZF (−Zn2+) and mono-ubiquitin (negative control). Error bars represent S.D. over two independent experiments. C, competition by unlabeled NEMO ZF of the binding of fluorescein-labeled NEMO ZF to mono-Ub. Fluorescein-labeled NEMO ZF (0.1 μm, 50 μl) was incubated for 30 min at 25 °C with mono-Ub (400 μm) in the presence of variable concentrations of unlabeled NEMO ZF (0–1000 μm). mP, millipolarization units.