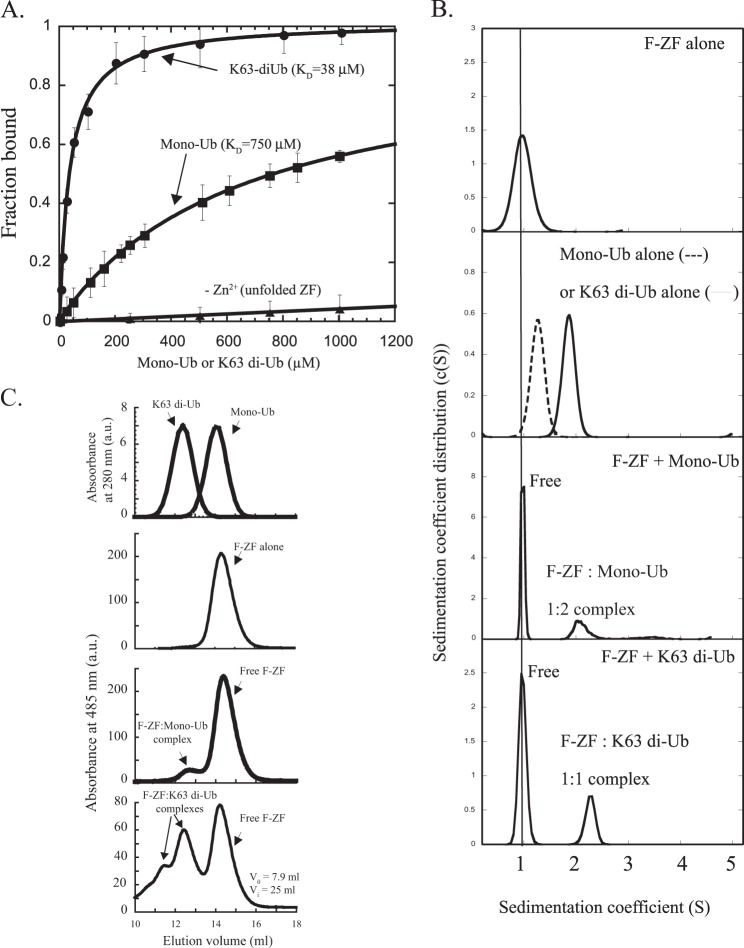
FIGURE 3.
The ZF UBD contains two ubiquitin binding sites that result in higher binding affinity to di-ubiquitin as compared with mono-ubiquitin. A, fluorescence polarization data for fluorescein-labeled NEMO ZF (F-ZF, 0.1 μm) interacting with mono-ubiquitin or Lys-63-di-ubiquitin. The dissociation constants (KD) were determined as described under “Experimental Procedures.” Error bars represent S.D. over two independent experiments. B, SV-AUC measured size distributions of fluorescein-labeled NEMO ZF in the presence or absence of mono-ubiquitin or Lys-63 di-ubiquitin. Sedimentation profiles of the free and bound states of NEMO ZF were monitored at 20 °C by absorbance at 485 nm, whereas those of mono- and di-ubiquitin alone were monitoring by absorbance at 280 nm or Rayleigh interference as described under “Experimental Procedures.” a.u., absorbance units. C, size exclusion chromatography analysis of NEMO F-ZF interactions with mono-ubiquitin or Lys-63-linked di-Ub. Fluorescein-labeled ZF (20 μm) was incubated in the absence or presence of mono-ubiquitin (Mono-Ub, 20 μm) or Lys-63-linked di-ubiquitin (K63 di-Ub, 20 μm), and the mixture was subjected to gel filtration analysis on a Superdex 75 HR 10/30 column at 4 °C. The amount of free or bound ZF formed upon incubation with mono-Ub or Lys-63 di-Ub was monitored at 485 nm by recording specific absorbance of the fluorescein-labeled ZF, whereas the elution profiles of mono-Ub and Lys-63 di-Ub alone were recorded at 280 nm as indicated. The void (V0) and internal (Vi) volumes of the column are indicated.