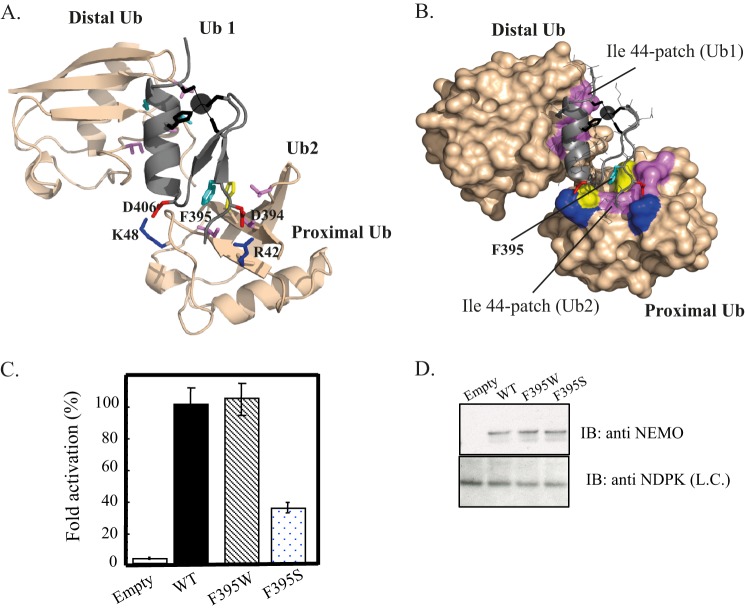
FIGURE 7.
Model of the NEMO ZF-Lys-63-di-ubiquitin complex. Ribbon (A) and surface (B) representations of the low energy structure of the NEMO ZF complex with Lys-63 di-Ub showing the second ubiquitin binding site (Ub2) and the prior ubiquitin binding site (Ub1) previously described in Ref. 15. The docking model was generated using the HADDOCK docking procedure. NEMO ZF is shown in gray, and Lys-63 di-Ub in light orange. The Phe-395, which forms the central hydrophobic core of Ub2, is colored in cyan and interacts with the Ile-44 patch of the proximal ubiquitin. Asp-406 and Asp-394 are shown in red and form salt bridges with Lys-48 and Arg-42 colored in blue. The zinc atom is represented as a black sphere and ligating residues (Cys-397, Cys-400, His-413, Cys-417) are represented as black sticks. The buried surface area of 1706 ± 56 A2 is 1.7-fold higher than the one calculated with the ZF-mono-Ub complex (1014 ± 55 Å2 (15)). C, NEMO-deficient Jurkat T cells were transiently co-transfected with the reporter plasmid Igκ-luciferase, a second reporter plasmid, EF1-β-galactosidase, to normalize transfections, and with an empty vector (Empty) or an expression vector encoding either WT, F395W, or F395S mutant. After transfection, cells were stimulated for 4 h with 20 ng/ml TNF-α. The NF-κB activation corresponding to the ratio of luciferase activity in the presence and in the absence of the stimulus is taken as 100% activity for WT. Error bars represent the S.D. values over three experiments. D, Western blot showing NEMO expression level in cell extracts of transiently transfected Jurkat T cells. Nucleotide diphosphate kinase (NDPK) was immunoblotted as a loading control (L.C.).