FIGURE 7.
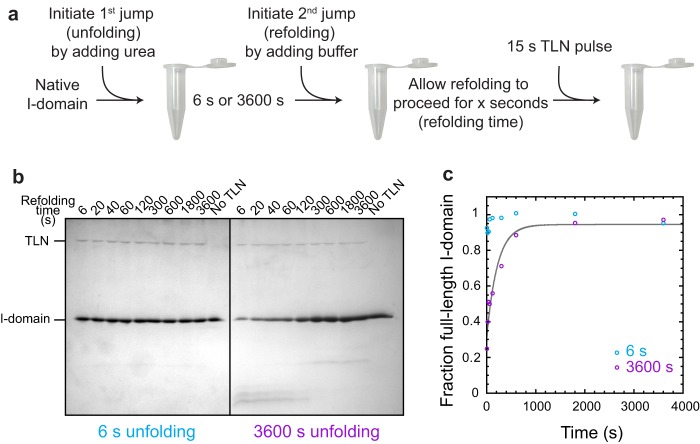
The I-domain folds immediately when the prolines are in the correct isomeric state. a, the I-domain was unfolded in 8 m urea for 6 or 3600 s, diluted into buffer, and then allowed to refold for varying times before being subjected to a 15-s pulse of digestion using a relatively high concentration of the nonspecific protease thermolysin. b, samples were quenched with sample buffer, and the extent of proteolysis was observed by 16% Tricine-SDS-PAGE. c, the full-length I-domain was quantified using densitometry and plotted versus refolding time. The solid gray line is the fit of the 3600-s unfolding data to a first-order rate equation with one exponential (see “Experimental Procedures”). The relaxation time from these data is included in Fig. 6.