FIGURE 1.
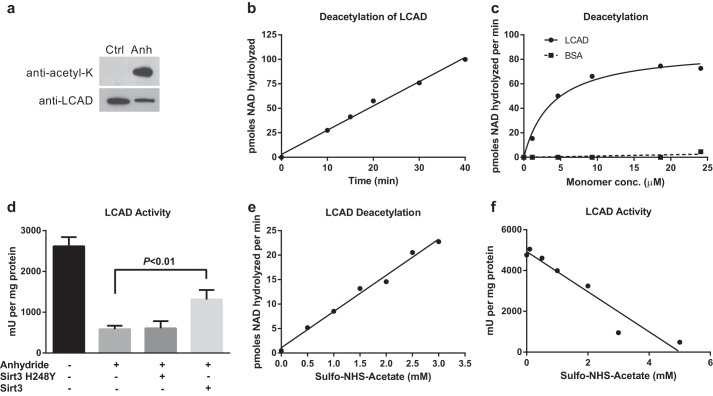
Chemically acetylated recombinant LCAD is a substrate for SIRT3. a, anti-acetyllysine Western blotting of acetic anhydride (Anh)- versus mock-treated recombinant LCAD protein, 20 ng/lane. Ctrl, control. b, 2.0 μm chemically acetylated LCAD was deacetylated by SIRT3 for the indicated time periods. Deacetylation was measured by following degradation of [14C]NAD+. Data points represent the average of duplicate assays. c, chemically acetylated LCAD and BSA were used as substrates for in vitro deacetylation assays with recombinant human SIRT3. Data points represent the average of duplicate assays. conc., concentration. d, anhydride acetylation of LCAD significantly reduces enzymatic activity. Deacetylation of anhydride-treated LCAD with SIRT3, but not the inactive mutant SIRT3-H248Y, partially restores enzymatic activity. Error bars represent mean ± S.D. of triplicate assays. e and f, sulfo-NHS-acetate was tested as an alternative to anhydride, which is chemically unstable. Incubation of LCAD with increasing concentrations of sulfo-NHS-acetate both increased the rate of SIRT3 deacetylation (e) and reduced the enzymatic activity of LCAD (f). Subsequent experiments utilized sulfo-NHS-acetate as an acetylating agent.