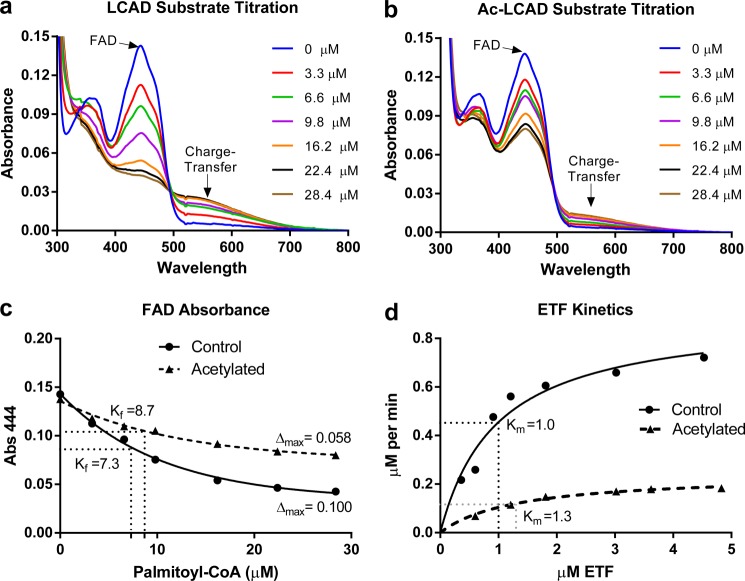
FIGURE 2.
Acetylation affects the active site of LCAD. Recombinant LCAD was either mock-treated (a) or acetylated with sulfo-NHS-acetate (Ac-LCAD) (b) and then titrated with palmitoyl-CoA under anaerobic conditions to study the reductive half-reaction. After each addition of substrate, the enzyme was allowed to stabilize for 10 s and then scanned on a spectrophotometer from 300–800 nm. The characteristic FAD peak (444 nm) becomes reduced, and the charge-transfer complex peak (∼570 nm) increases with increasing substrate concentrations. c, the absorbance (Abs.) of the FAD peak (444 nm) from the curves in a and b were plotted, fit with nonlinear regression, and used to calculate Δmax (maximum change in absorbance) and Kf (apparent substrate binding constant, in micromolar). d, the oxidative half-reaction was studied by kinetic assays with increasing concentrations of the physiological electron acceptor ETF. Data points represent the average of duplicate assays. The curves were fit with non-linear regression (Michaelis-Menten) and used to calculate Km, Vmax, and catalytic efficiency.