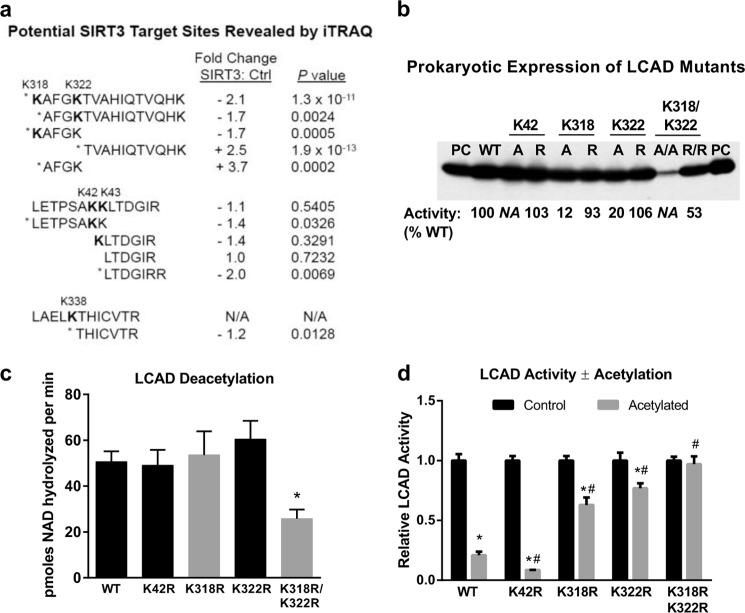
FIGURE 3.
LCAD residues Lys-318 and Lys-322 are targets of SIRT3 and modulate enzymatic activity. a, chemically acetylated LCAD was either mock-treated or SIRT3-treated in quadruplicate. The protein samples were digested with trypsin, labeled with 8-plex iTRAQ isobaric tags, and subjected to LC-MS/MS. The relative abundance of eight peptides changed significantly with SIRT3 incubation. Three of these were acetylated peptides (Lys-42, Lys-318, and Lys-322), and five were unmodified peptides. Some peptides with non-significant change are shown for context. *, p < 0.05; SIRT3-treated versus control; N/A, not applicable, this peptide was not quantified in the iTRAQ assay. b, the three lysines identified by iTRAQ proteomics (Lys-42, Lys-318, and Lys-322) were mutated to either alanine (A) or arginine (R) and expressed in E. coli. Crude lysates (5 μg) were Western-blotted with anti-LCAD antibody. 10 ng of purified LCAD was run as positive control (PC). Subsequently, six of these mutant proteins were purified to homogeneity (K42R, K318A, K318R, K322A, K322R, and K318R/K322R), and enzymatic activity was measured in triplicate. Activities are shown as a percentage of WT LCAD activity below the Western blot. N/A, not applicable. These proteins were not purified and thus were not assayed. c, the purified arginine mutant LCAD proteins were acetylated with sulfo-NHS-acetate and used as substrates for SIRT3 deacetylation assays. Shown is the mean ± S.D. of triplicate 20-min deacetylation assays using 19 μm LCAD monomer. *, p < 0.001 versus the wild type. d, samples of wild-type LCAD and arginine mutants were acetylated with sulfo-NHS-acetate and tested for enzymatic activity versus mock-treated enzyme. For ease of comparison, the activity of each mock-treated enzyme was set to 1.0. Note that only K318R/K322R had reduced starting activity compared with WT (b). Shown is the mean ± S.D. of triplicate activity assays. *, p < 0.001, acetylated enzyme versus the corresponding non-acetylated control; #, p < 0.001 acetylated mutant LCAD versus acetylated wild-type LCAD.