FIGURE 5.
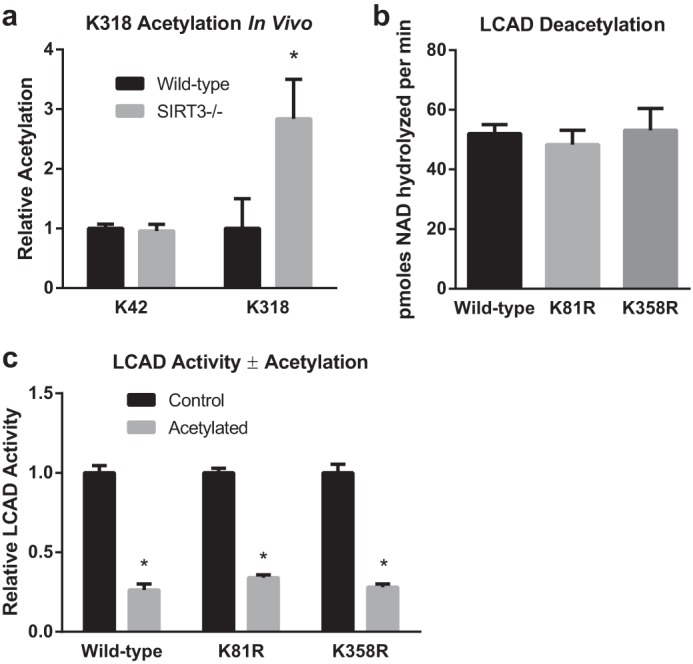
LCAD Lys-318 is a SIRT3 target site in vivo, whereas Lys-81 and Lys358 do not appear to play a role in the regulation of LCAD activity by acetylation. a, SRM proteomics were used to determine the abundance of the acetylated peptide KAFGK (acetylated at Lys-318) in liver mitochondria isolated from wild-type and SIRT3 knockout mice (n = 5). As a control, the abundance of the acetylated peptide LETPSAKK was determined. Samples were supplemented with isotopically labeled LETPSAKK* (acetylated at Lys-42, and where the C-terminal residue is 13C6,15N2-lysine), and the resulting heavy peptide signal was used for normalization. Shown is the mean ± S.D. *, p < 0.05. b and c, the importance of Lys-81 and Lys-358 as potential SIRT3-targeted sites was evaluated by creating K81R and K358R mutant LCAD proteins, chemically acetylating them with sulfo-NHS-acetate, and testing the resulting acetylated proteins as substrates for SIRT3 (b) and for enzymatic activity (c). The K81R and K358R substitutions did not affect the rate of deacetylation or provide protection against acetylation-induced loss of activity. Shown is the mean ± S.D. of triplicate assays. *, p < 0.001 for acetylated versus control proteins.
