FIGURE 9.
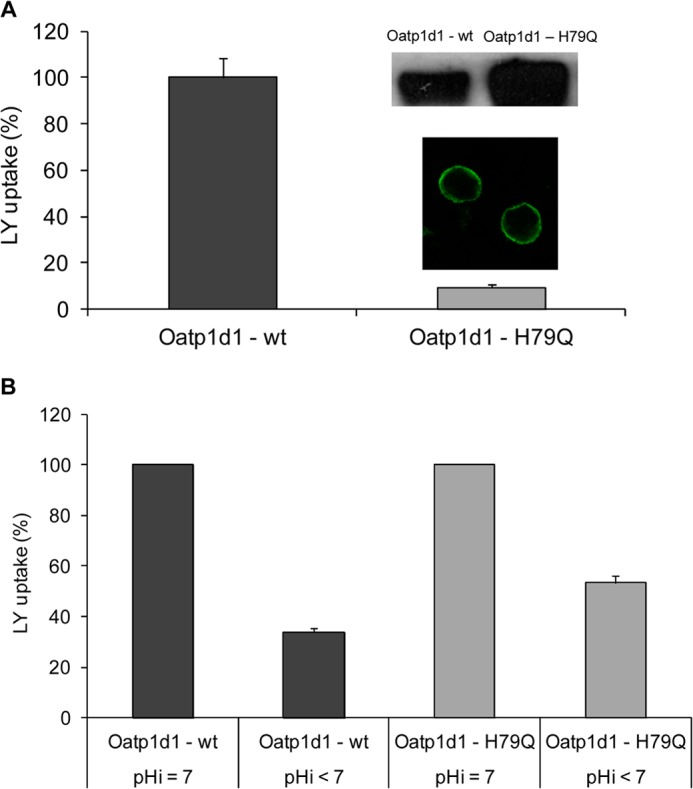
Histidine at position 79 is crucial for the Oatp1d1 transport activity, and its protonation is partly responsible for the reduction of Oatp1d1 transport activity at alkaline extracellular pH. A, activity of the Oatp1d1-H79Q mutant shown as a percentage of LY uptake relative to the Oatp1d1-WT. Uptake of LY by Oatp1d1 and Oatp1d1-H79Q was measured in transiently transfected HEK293 cells after a 15-min incubation with 100 μm LY at 37 °C. Data represent mean ± S.E. (error bars) of three independent experiments. Inset, localization of the H79Q mutant in the plasma membrane obtained by immunofluorescence analysis by confocal microscopy and high protein levels on Western blots stained with anti-His antibody. Immunocytochemistry was performed with FITC antibody. B, change in activity of Oatp1d1-WT and Oatp1d1-H79Q at neutral and acidified pHi. Results represent the percentage of LY uptake relative to Oatp1d1-WT at neutral pHi and relative to Oatp1d1-H79Q at neutral pHi, respectively. Data represent mean and S.E. from triplicate determinations.
