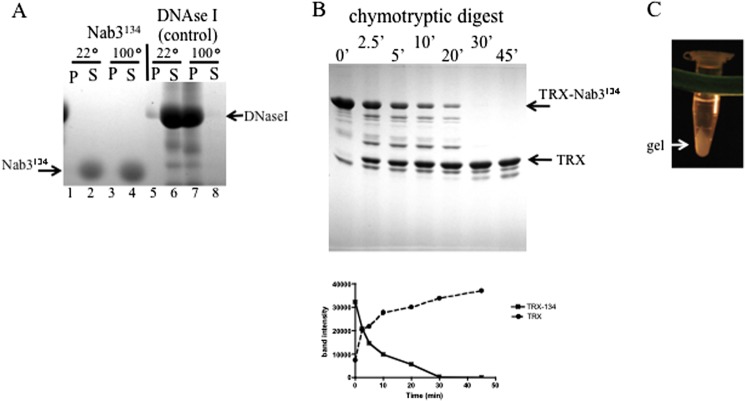
FIGURE 4.
Characterization of the 134-amino acid Nab3 peptide. A, heat stability. Forty μg of isolated Nab3 134-amino acid peptide or deoxyribonuclease I (Stratagene) were incubated at 22 or 100 °C for 45 min and separated into insoluble (P) and soluble (S) fractions by centrifugation. Samples were resolved on 11% SDS-polyacrylamide gels and stained with Coomassie Blue. B, time course of chymotryptic digestion of the Nab3 134-amino acid peptide fused to thioredoxin (TRX-Nab3134). Chymotrypsin was incubated with the target protein at a molar ratio of 1:400 for the indicated times before reactions were stopped and resolved on SDS-PAGE. Graphed below is a quantification of the stained bands using ImageJ software, showing loss of the full-length fusion protein (TRX-Nab3134) and accumulation of the thioredoxin tag (TRX) over time. C, gel formation. After incubation of purified Nab3134 at 4 °C, a hydrogel could be detected with the naked eye, and it is shown here after pelleting in a microcentrifuge.