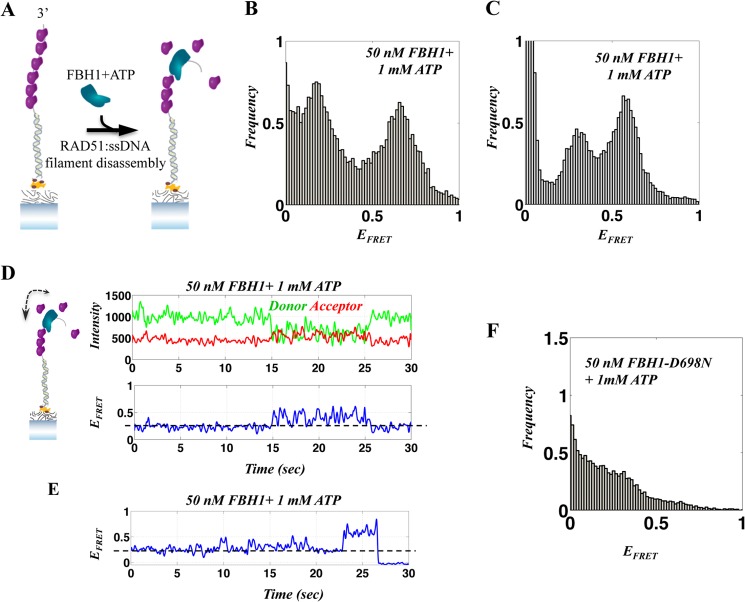
FIGURE 8.
Single-molecule FRET analysis of the FBH1-RAD51 interaction. A, illustration of the DNA FRET assay for single-molecule, FBH1-mediated, RAD51-ssDNA filament disassembly. The addition of FBH1 to a preformed RAD51 filament causes filament disassembly enabling relaxation (shortening) of ssDNA and an increase in FRET efficiency. B and C, FRET histograms after the addition of FBH1 and ATP to a preformed RAD51K133R-ssDNA filament for the 20-nt (B) and 30-nt (C) substrates. D and E, representative smFRET trajectories of the dynamics associated with repetitive translocation of FBH1 on ssDNA in the presence of a RAD51K133R filament and FBH1-mediated RAD51K133R-ssDNA filament disassembly. The FRET efficiency corresponding to a RAD51K133R-ssDNA filament (dashed line) displays changes due to repetitive FBH1 ssDNA translocation that fluctuate around the filament base line (D), indicating the co-existence of FBH1 and the RAD51K133R filament. E, fluctuations of FRET corresponding to FBH1 translocations in the presence of RAD51K133R-ssDNA filament, followed by an abrupt increase to higher FRET due to the removal of RAD51K133R filament. F, FRET histogram after the addition of FBH1-D698N mutant (ATPase-dead) and ATP to a preformed RAD51K133R-ssDNA filament for the 20-nt DNA, showing the persistence of the RAD51K133R filament.