FIGURE 4.
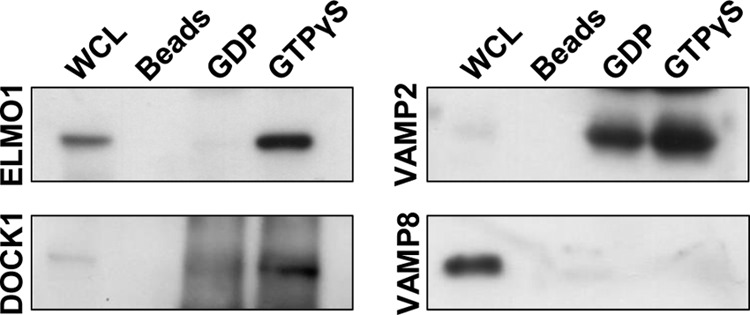
GST-RhoG fusion protein loaded with either GDP or GTPγS was used to pull down potential interacting proteins from human platelet whole cell lysates (WCL). Candidate interacting proteins were initially identified by mass spectrometry. Immunoblotting was then used to confirm the specificity of these interactions. Only active (GTPγS-bound) RhoG bound ELMO1 and DOCK1 (DOCK180). VAMP2 interacted with both GDP- and GTPγS-loaded RhoG, but there was greater binding to the active form. RhoG interacted only with certain SNARE proteins, however; for instance, RhoG did not interact with VAMP8 in either the GDP- or GTP-loaded state. GSH-Sepharose beads were used to control for nonspecific binding. None of these proteins bound to the beads themselves.
