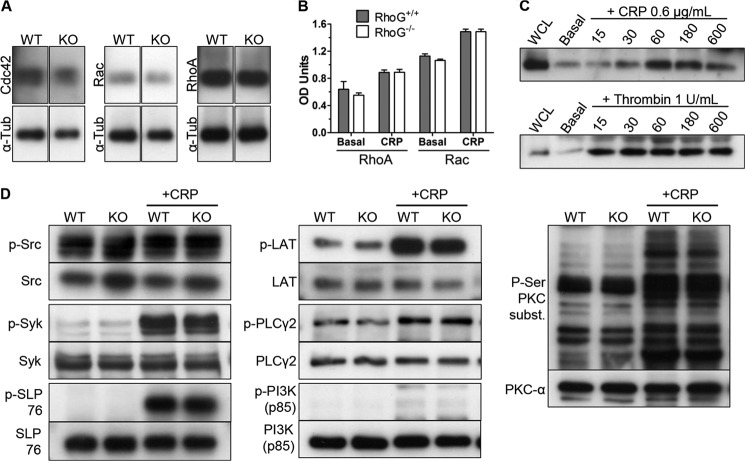
FIGURE 5.
A, immunoblotting for other Rho family GTPases in WT and RhoG−/− platelets demonstrated that there was no reduction in expression of Cdc42, Rac, or RhoA in RhoG−/− platelets. KO, knock-out; α-Tub, α-tubulin. B, plate-based G-LISA assays were used to evaluate the activation of RhoA and Rac in WT and RhoG−/− platelets in response to CRP. Stimulation of washed platelets with CRP (0.6 μg/ml) activated Rac and RhoA, but no differences in the activity of either GTPase was noted in basal or post-stimulation samples from RhoG−/− platelets compared with WT platelets, suggesting that as in lymphocytes, RhoG does not act via Rac in platelets. Data are presented as means ± S.E. of three mice/group for each condition. C, the activation of RhoG in mouse platelets in response to CRP and thrombin stimulation was assessed using a GST-ELMO pulldown assay. In mouse platelets, RhoG was activated by both CRP and thrombin, although at the concentrations used, RhoG was activated more slowly in response to CRP compared with thrombin. WCL, whole cell lysate. D, the role of RhoG in the tyrosine kinase signaling pathway downstream of GPVI was evaluated using phosphorylation-specific antibodies. Washed platelets from WT and RhoG−/− mice in basal states and following stimulation with 0.6 μg/ml CRP for 60 s were lysed and prepared for immunoblotting. Phosphorylation of Src family kinases (Tyr-416), Syk (Tyr-525/Tyr-526), SLP76 (Tyr-128), PI3K p85 (Tyr-458), LAT (Tyr-191), PLCγ2 (Tyr-759), and the substrates of PKC (phospho-Ser) was assessed. Immunoblots using antibodies against the non-phosphorylated forms were used as loading controls. No change in Src family kinase phosphorylation status was noted, but for the other kinases assessed, phosphorylation increased upon CRP stimulation. No differences between the WT and RhoG−/− platelets were seen in basal or stimulated samples, however. Immunoblots are representative of at least three separate experiments.