Abstract
Objectives: The aim of this study was to investigate if there is any interaction between ondansetron and nefopam when they are continuously co-administrated during patient-controlled intravenous analgesia (PCIA).
Methods: The study was a prospective, randomized, controlled, non-inferiority clinical trial comparing nefopam-plus-ondansetron to nefopam alone. A total of 230 postoperative patients using nefopam for PCIA, were randomly assigned either to a group receiving continuous infusion of ondansetron (Group O) or to the other group receiving the same volume of normal saline continuously (Group N). Postoperative pain intensity scores, the sum of pain intensity difference over 24 hours postoperatively (SPID24hr), the incidence of adverse events, and the total consumption of nefopam were evaluated respectively.
Results: Postoperative pain was treated successfully in both groups. The mean SPID24hr scores were 95.6 mm in Group N and 109.3mm in Group O [95% confidence interval (CI) -14.28, 24.32]. The lower margin of the 95% CI was above the pre-determined non-inferiority margin (-30mm) for SPID24hr, which indicated that nefopam-plus-ondansetron was not worse than the nefopam alone in term of analgesic efficacy. In addition, there was no statistical difference between the two groups in term of cumulative consumption of nefopam. Compared with Group N, postoperative vomiting was significantly reduced in Group O during the postoperative 24 hours (P < 0.05). Less rescue antiemetics were given to patients in Group O than those receiving nefopam alone (P < 0.05). There were no differences in postoperative nausea between the two groups.
Conclusion: Nefopam-plus-ondansetron is not inferior to nefopam alone in relieving the pain in PCIA after minimally invasive surgery. In addition, adverse events are reduced without compromising analgesic efficacy.
Keywords: Postoperative pain, Postoperative nausea and vomiting, Ondansetron, Nefopam.
Introduction
Postoperative pain management is critical during patients' recovery 1-3. Nefopam is a clinically effective analgesic agent that is frequently used to control mild to moderate pain 4-6. The analgesic effect of nefopam involves (i) inhibition of glutamatergic pathway by modulating calcium and sodium channels 7, 8; (ii) inhibition of serotonin (5-hydroxytryptamine, 5-HT) and noradrenaline reuptake in the central nervous 9, 10. In clinical practice, concomitant use of nefopam and antiemetic agent postoperatively is a possible way to attenuate vomiting which is an adverse effect of nefopam appearing in 15 - 30% of treated patients 4.
Ondansetron, a selective serotonin subtype 3 (5-HT3) receptors antagonist, is currently used for prophylaxis and treatment of postoperative nausea and vomiting (PONV) in perioperative management 11, 12. Some studies have revealed that ondansetron can reduce or block the antinociceptive effect of certain classical analgesics, such as paracetamol 13, 14 and tramadol 15, 16, with diverse and unknown mechanisms of action. However, little is known about the effect of ondansetron on the antinociceptive efficacy of nefopam.
Randomized controlled non-inferiority study was carried out to test the hypothesis whether the combination of nefopam and ondansetron would lead to mutually contrasting modifications of serotonergic transmission mediated by 5-HT3 receptors, and moreover, whether ondansetron-induced antagonism of 5-HT3 receptors could modify the antinociceptive effect of nefopam.
Methods and Materials
After obtaining the ethic approval from the Ethics Committee of Southwest Hospital, 230 patients (American Society of Anaesthesiologists physical status I or II), whose age were between 30 and 60 years old and scheduled for elective unilateral percutaneous nephrolithotomy (PCNL) surgery, were enrolled in this study. Written informed consent was obtained from each patient. Exclusion criteria were as follows: contraindications for the use of nefopam or ondansetron use; pregnant or lactating women; preoperative use of antiemetic, antidepressant or analgesics; history of motion sickness, epilepsy, or alcohol abuse; severe cardiac disease; renal or hepatic insufficiency. Patients were all unpremedicated.
On the day before surgery, the use of the patient-controlled intravenous analgesia (PCIA) device and of the 100-mm visual analog scale (VAS) (0 mm = no pain, 100 mm = worst imaginable pain) for assessing postoperative pain intensity 4 was explained to every patients.
Anaesthesia
Identical anesthetics and techniques were used for all the patients. General anesthesia was induced intravenously with fentanyl (3μg/kg), midazolam (0.05mg/kg) and propofol (2mg/kg). Then vecuronium (0.1mg/kg) was given to facilitate orotracheal intubation. After endotracheal intubation, general anesthesia was maintained with 1.5 MAC of sevoflurane in 60% nitrous oxide/oxygen; continuous-infusion remifentanil (0.2µg/kg) was used for intraoperative analgesia. Supplementary doses of vecuronium were administered wherever necessary. Mechanical ventilation was adjusted to maintain the end-tidal of PCO2 at 4.5∼5.0kPa. Heart rate, non-invasive blood pressure and pulse oximetry were monitored continuously using Marquette Eagle 4000 monitor (GE, New York, USA) throughout the anesthesia and surgery. Ringer's solution was infused at 10 ml · kg-1 · h-1.
At the end of the surgery, inhalational anesthesia was discontinued and a loading dose of nefopam (20mg) was administered to all the patients. Residual neuromuscular blockade was reversed with neostigmine (0.04mg/kg) and atropine (0.02mg/kg) when required. The trachea was extubated when respiration was adequate and the patient responded to verbal commands. All patients were transferred to the postanesthesia care unit (PACU) after extubation.
Postoperative care
In the PACU, patients were connected to a PCIA device (AutoMed 3300 PCA infusion pump; ACE Medical Company, Kyungi, Korea) and a balloon infuser (Accufuser, 2ml/h; Wooyoung Medical Co., Ltd., Paju, Korea). Using a computerized randomization table, patients were assigned to two groups: Group N (nefopam and saline) or Group O (nefopam and ondansetron). PCIA pumps was filled with nefopam (1.6mg/ml) in saline solution and set with a bolus of 1ml (corresponding with nefopam 1.6mg demand dose), a 10-min lockout interval, a continuous background infusion of 2ml/h, and a 4-hour maximal dose of 30mg. In Group N, the balloon device was filled with 48ml of saline. In Group O, the elastomer contained ondansetron (0.5mg/ml) in saline (total volume, 48ml), with a drug infusion rate of 2ml/h. All drugs and devices were prepared by an anesthetist who was not involved in the management of the patients.
Postoperative pain intensity was evaluated by assessment of pain score on the VAS. Two anesthesiologists, who were blinded with the study protocol, evaluated and recorded the VAS scores at the 0th, 6th, 12th, and 24th hour postoperatively, as well as the respiratory depression, postoperative nausea and vomiting, and the cumulative dose of nefopam consumption during postoperative 24 hours. Any patients who did not obtain satisfactory pain relief from the above PCIA regimen were excluded from further study and then received a single i.v. 50µg dose of fentanyl. For patient with severe nausea and vomiting, an intravenous dose of metoclopramide (generally at 10mg, up to a maximum of 30mg) was administered.
Outcomes
The primary outcome variable was the sum of pain intensity difference (PID) over 24 hours postoperatively (SPID24hr) assessed by 0 - 100 mm VAS for the per-protocol population. PID was calculated by subtracting current pain intensity from baseline pain intensity in order to examine the change in pain intensity from the baseline. The SPID24hr for each subject was the sum of the PID at 6 hours (VAS at baseline - VAS at 6 hours), at 12 hours (VAS at baseline - VAS at 12 hours) and at 24 hours (VAS at baseline - VAS at 24 hours). This represented the total reduction in pain intensity over 24 hours.
The secondary outcome variables included: 1) pain intensity assessed through VAS scores at the 0th, 6th, 12th, and 24th hour postoperatively; 2) the cumulative dose of nefopam consumption in postoperative 24 hours; 3) the number of patients requiring rescue medication during postoperative 24 hours; 4) the frequency of adverse events during postoperative 24 hours.
Statistical analysis
The primary aim of the study was to show that the combination of nefopam and ondansetron is not inferior to the nefopam alone in term of the treatment of postoperative pain. Based on a standard deviation of 60 mm determined by preliminary experiment and experience, a sample size of 222 patients (111 patients per group) was calculated using NCSS-PASS (NCSS LLC, Kaysville, USA), with a Type I error rate of 0.05. Then our statistical study had 80% power to reject the null hypothesis (nefopam-plus-ondansetron is worse than the nefopam alone) and accept the alternative hypothesis of non-inferiority. 115 patients were included in each group in case there were expulsion cases. Non-inferiority would be declared for nefopam-plus-ondansetron if the lower bound of the two-side 95% confidence interval (CI) of the SPID24hr difference between the two treatment groups was greater than the pre-specified non-inferiority margin (-30mm).
Statistical analysis was performed by SPSS version 17.0 (SPSS Inc, Chicago, Illinois, USA). Data distribution was evaluated with the Shapiro-Wilk's test. Parametric data were analyzed by two-tailed independent t-test. Nonparametric data were assessed by Wilcoxon signed rank sum test. Category data were analyzed by Chi-square test or Fisher's exact test, as appropriate. Values were reported as mean ± SD, absolute number (n), or percentages. A P-value <0.05 was set as statistically significant.
Results
All the 230 patients received their assigned treatments. There were no significant differences between the groups regarding patient demographic characteristics, ASA physical status, duration of surgery, starting time of PICA, and baseline of pain intensity scores (Table 1).
Table 1.
Demographic Characteristics and Perioperative Data
| Group N (n=115) | Group O (n=115) | |
|---|---|---|
| Age (yr) | 49 ± 9 | 53 ± 10 |
| Weight (kg) | 63.3 ± 9.4 | 67.8 ± 10.1 |
| Gender (M/F) | 64/51 | 73/42 |
| ASA I/II | 93/22 | 102/13 |
| Duration of surgery (min) | 78 ± 23 | 71 ± 17 |
| Starting time of PCIA (min) | 28 ± 17 | 24 ± 20 |
| Baseline pain intensity (VAS scores at postoperative 0 hour), n (%) | ||
| Slight pain (10 - 30 mm) | 26 (22.6%) | 21 (18.3%) |
| Moderate pain (40 - 60 mm) | 82 (71.3%) | 89 (77.4%) |
| Severe pain (70 - 100 mm) | 7 (6.1%) | 5 (4.3%) |
ASA: American Society of Anaesthesiologists physical status; PCIA: patient-controlled intravenous analgesia; VAS: visual analog scale.
Data are presented as mean ± SD, number or number (percent).
No statistically significant between-group differences (P ≥ 0.05).
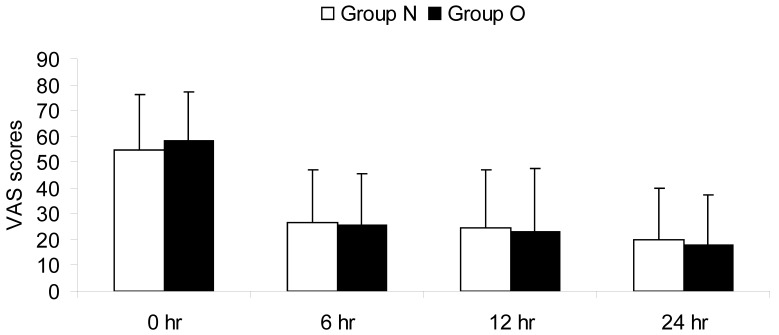
Postoperative pain was treated successfully in both groups. At the 6th, 12th, 24th hour postoperatively, pain scores were never more than 40 mm according to a VAS. No statistical significant differences were found between the two groups with regard to pain VAS scores at any time point (Fig.1).
Figure 1.
Pain intensity scores during the postoperative 24 hours. VAS: visual analog scale; 0 hr: the 0th hour after surgery; 6 hr: the 6th hour after surgery; 12 hr: the 12th hour after surgery; 24 hr: the 24th hour after surgery.
SPID24hr, the primary outcome variable, was considered as the non-inferiority criterion. The mean SPID24hr score was 95.6 mm in Group N, and 109.3mm in Group O. The SPID24hr difference was the difference between the nefopam-plus-ondansetron and the nefopam alone. The upper and lower limits of the 95% CI of the SPID24hr difference were 24.32 and -14.28 respectively (Table 2). Clearly, the lower limit of the 95% CI was above the pre-determined non-inferiority margin (-30mm) for SPID24hr, which indicated that nefopam-plus-ondansetron is not worse than nefopam alone in relieving the postoperative pain.
Table 2.
SPID24hr by VAS scores and the consumption of analgesic during the postoperative 24 hours
| Group N (n=115) | Group O (n=115) | |
|---|---|---|
| SPID24hr (mm) | 95.6 ± 57.4 | 109.3 ± 64.1 |
| 95% CI of the SPID24hr difference | -14.28, 24.32 | |
| consumption of nefopam during the postoperative 24 hours (mg) | 98 ± 12.4 | 104 ± 17.6 |
SPID24hr: the sum of pain intensity difference over 24 hours postoperatively; VAS: visual analog scale.
Data are presented as mean ± SD.
In term of the cumulative consumption of nefopam, there was no statistical difference between the two groups (Table 2). Note that no patient was excluded from the study in both groups as none of them asked for analgesic rescue due to inadequate analgesia.
Postoperative vomiting was significantly more common in Group N (15.7%) compared with that in Group O (3.5%) during the postoperative 24 hours (P < 0.05) (Table 3). Patients were given significantly more rescue antiemetics in Group N (12.2%) compared with that in Group O (2.6%) (P < 0.05). However, there was no difference in postoperative nausea between the two groups. Incidence of respiratory depression did not occur in patients in both groups.
Table 3.
Incidence of adverse events during the postoperative 24 hours
| Group N (n=115) | Group O (n=115) | |
|---|---|---|
| Nausea, n (%) | 34 (29.6%) | 38 (33%) |
| Vomitting, n (%) | 18 (15.7%)* | 4 (3.5%) |
| Need for rescue antiemetics, n (%) | 14 (12.2%)* | 3 (2.6%) |
| Respiratory depression, n (%) | 0 | 0 |
Data are presented as number (percent).
* P < 0.05 compared with the Group O.
Discussion
The non-opioid analgesic nefopam is often used for pain management for postoperation patient 4, 5. It has been proved that nefopam is effective in different routes of administration 17, but to date, its efficacy in PCIA remains unclear. In our study, it is the first time to show that administration of nefopam in PCIA can provide efficacious and safe analgesia to patients after PCNL. Moreover, it is found that there is no antagonistic interaction between ondansetron and nefopam and thus the combination of them is safe to be used to ease pain.
As a minimally invasive surgery, PCNL has been widely accepted. Several new techniques of PCNL, such as mini-PCNL and tubeless PCNL, have been reported to decrease the morbidity and analgesic requirement, but even then it is still a painful procedure. Tangpaitoon and colleagues 18 had reported that analog pain score (VAS scores) at the postoperative 1 hour and 4 hour were 68.8 ± 12.7 mm and 50.7 ± 25.8 mm, respectively, for patients who underwent PCNL with general anesthesia. Singh et al. 19 had reported that VAS on the first postoperative day morning was 65.6 ± 14.4 mm in the general anesthesia patients. Thus, it is clear that the postoperative administration of analgesic agents is necessary in patients undergoing PCNL.
According to our results, the pain intensity scores were never more than 40 mm referring to a VAS for all of the patients and no rescue analgesic was required. This implies that the postoperative pain can be treated successfully by nefopam in the PCIA route, even if the efficacy of nefopam seems lower than that of opioids 17. The present study provides a possibility to make use of nefopam in PCIA for post-minimally invasive surgery analgesia in clinical practice.
Serotonin plays a key role in pain control mechanisms 20 and multiple serotonin receptor subtypes (5-HT1, 5-HT2 and 5-HT3 receptors) are now thought to be involved in the serotonin-mediated antinociceptive mechanism 21. Although the antinociceptive mechanism of action of nefopam is largely unknown, inhibition of the serotonin reuptake in synaptosome and activation of descending serotonergic pathways have been demonstrated 6. A previous animal study has shown that concomitant use of the serotonin depletory para-chlorophenylalanine with nefopam completely blocks the analgesic effect of nefopam in the mouse formalin test 22. The authors suggest that the effect of para-chlorophenylalanine results from a pharmacodynamic interaction, implying that the central serotonergic pathways may be involved in the mechanism of action of nefopam 22. Nefopam permits endogenous serotonin to exert its antinociceptive effect. Hence, it is theoretically possible that the serotonin receptor antagonists could enhance or weaken the antinociception exerted by nefopam. However, few studies have been performed on the pharmacological interaction between nefopam and serotonin 5-HT3 receptor antagonists (dolasetron, granisetron, ondansetron, palonosetron, tropisetron) in human. Moreover, ondansetron is increasingly used for prophylaxis of postoperative nausea and vomiting in perioperative management 11, 12, whereas tropisetron and granisetron are used only for chemotherapy nausea 23. This is the reason why the interaction between nefopam and ondansetron was assessed in the present study.
Our results indicate that there is no change in the antinociceptive effect of nefopam when ondansetron is co-administered. These findings can be explained as follows: First of all, it has been demonstrated in animal model of pain that ondansetron has little antagonistic effect on the analgesic efficacy of nefopam. Girard and colleagues 24 have demonstrated that 5-HT1B and 5-HT2C, but not 5-HT3, receptor subtypes are involved in the antinociceptive effect of nefopam. These results imply that the serotonergic system implicated in the analgesia exerted by nefopam may involve only specific serotonin receptor subtypes (5-HT1B and 5-HT2C) but not all (e.g. 5-HT3). Secondly, ondansetron is a substrate of the phosphoglycoprotein (P-gp) transport pump encoded by the MDR1a gene 25. Under normal conditions, ondansetron is actively pumped out of the central nervous system across blood-brain barrier against concentration gradient 26. We consider the possibility that the lack of the change in the antinociceptive effect of nefopam may result from the failure of ondansetron accumulation in the central nervous system to sufficient concentration due to the extrusion by P-gp transport pump. The results in this study suggest that it may be possible to use ondansetron as antiemetics with nefopam during the perioperative period for analgesia.
The higher incidence of vomiting in Group N during the postoperative 24 hours seems to be correlated to the side effects of nefopam. Indeed, ondansetron is likely to reduce the incidence of vomiting induced by nefopam administration significantly, even though it has very little effect on the incidence of nausea 27.
In conclusion, the present study provides the first evidence that administration of nefopam in PCIA can provide efficacious and safe postoperative analgesia to patients after minimally invasive surgery. Moreover, comparing with using nefopam alone, nefopam plus ondansetron can reduce gastrointestinal adverse events without compromising analgesic efficacy.
References
- 1.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921–1928. doi: 10.1016/S0140-6736(03)14966-5. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet F, Marret E. Influence of anaesthetic and analgesic techniques on outcome after surgery. Br J Anaesth. 2005;95:52–58. doi: 10.1093/bja/aei038. [DOI] [PubMed] [Google Scholar]
- 3.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 4.Du Manoir B, Aubrun F, Langlois M, Le Guern ME, Alguier C, Chauvin M, Fletcher D. Randomized prospective study of the analgesic effect of nefopam after orthopaedic surgery. Br J Anaesth. 2003;91:836–841. doi: 10.1093/bja/aeg264. [DOI] [PubMed] [Google Scholar]
- 5.Kapfer B, Alfonsi P, Guignard B, Sessler DI, Chauvin M. Nefopam and ketamine comparably enhance postoperative analgesia. Anesth Analg. 2005;100:169–174. doi: 10.1213/01.ANE.0000138037.19757.ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beloeil H, Delage N, Negre I, Mazoit JX, Benhamou D. The median effective dose of nefopam and morphine administered intravenously for postoperative pain after minor surgery: a prospective randomized double-blinded isobolographic study of their analgesic action. Anesth Analg. 2004;98:395–400. doi: 10.1213/01.ANE.0000093780.67532.95. [DOI] [PubMed] [Google Scholar]
- 7.Verleye M, Andre N, Heulard I, Gillardin JM. Nefopam blocks voltage-sensitive sodium channels and modulates glutamatergic transmission in rodents. Brain Res. 2004;1013:249–255. doi: 10.1016/j.brainres.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Novelli A, Diaz-Trelles R, Groppetti A, Fernandez-Sanchez MT. Nefopam inhibits calcium influx, cGMP formation, and NMDA receptor-dependent neurotoxicity following activation of voltage sensitive calcium channels. Amino Acids. 2005;28:183–191. doi: 10.1007/s00726-005-0166-0. [DOI] [PubMed] [Google Scholar]
- 9.Gray AM, Nevinson MJ, Sewell RD. The involvement of opioidergic and noradrenergic mechanisms in nefopam antinociception. Eur J Pharmacol. 1999;365:149–157. doi: 10.1016/s0014-2999(98)00837-1. [DOI] [PubMed] [Google Scholar]
- 10.Rosland JH, Hole K. The effect of nefopam and its enantiomers on the uptake of 5-hydroxytryptamine, noradrenaline and dopamine in crude rat brain synaptosomal preparations. J Pharm Pharmacol. 1990;42:437–438. doi: 10.1111/j.2042-7158.1990.tb06587.x. [DOI] [PubMed] [Google Scholar]
- 11.Diemunsch P, Gan TJ, Philip BK, Girao MJ, Eberhart L, Irwin NG, Pueyo J, Chelly JE, Carides AD, Reiss T, Evans JK, Lawson FC, Aprepitant-PONV Protocol 091 International Study Group. Single-dose aprepitant vs ondansetron for the prevention of postoperative nausea and vomiting: a randomized, duble-blind phase III trial in patients undergoing open abdominal surgery. Br J Anaesth. 2007;99:202–211. doi: 10.1093/bja/aem133. [DOI] [PubMed] [Google Scholar]
- 12.Rosow CE, Haspel KL, Smith SE, Grecu L, Bittner EA. Haloperodol versus ondansetron for prophylaxis of postoperative nausea and vomiting. Anesth Analg. 2008;106:1407–1409. doi: 10.1213/ane.0b013e3181609022. [DOI] [PubMed] [Google Scholar]
- 13.Libert F, Bonnefont J, Bourinet E, Doucet E, Alloui A, Hamon M, Nargeot J, Eschalier A. Acetaminophen: a central analgesic drug that involves a spinal tropisetron-sensitive, non-5-HT(3) receptor-mediated effect. Mol Pharmacol. 2004;66:728–734. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 14.Pickering G, Loriot MA, Libert F, Eschalier A, Beaune P, Dubray C. Analgesic effect of acetaminophen in humans: first evidence of a central serotonergic mechanism. Clin Pharmacol Ther. 2006;79:371–378. doi: 10.1016/j.clpt.2005.12.307. [DOI] [PubMed] [Google Scholar]
- 15.Arcioni R, della Rocca M, Romano S, Romano R, Pietropaoli P, Gasparetto A. Ondansetron inhibits the analgesic effects of tramadol: a possible 5-HT(3) spinal receptor involvement in acute pain in humans. Anesth Analg. 2002;94:1553–1557. doi: 10.1097/00000539-200206000-00033. [DOI] [PubMed] [Google Scholar]
- 16.Dursteler C, Masea A, Fernandez V, Pol O, Puig MM. Interaction between tramadol and two anti-emetics on nociception and gastrointestinal transit in mice. Eur J Pain. 2006;10:629–638. doi: 10.1016/j.ejpain.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Evans MS, Lysakowski C, Tramer MR. Nefopam for the prevention of postoperative pain: quantitative systematic review. Br J Anaesth. 2008;101:610–617. doi: 10.1093/bja/aen267. [DOI] [PubMed] [Google Scholar]
- 18.Tangpaitoon T, Nisoog C, Lojanapiwat B. Efficacy and safety of percutaneous nephrolithotomy (PCNL): a prospective and randomized study comparing regional epidural anesthesia with general anesthesia. Int Braz J Urol. 2012;38:504–511. doi: 10.1590/s1677-55382012000400010. [DOI] [PubMed] [Google Scholar]
- 19.Singh V, Sinha RJ, Sankhwar SN, Malik A. A prospective randomized study comparing percutaneous nephrolithotomy under combined spinal-epidural anesthesia with percutaneous nephrolithotomy under general anesthesia. Urol Int. 2011;87:293–298. doi: 10.1159/000329796. [DOI] [PubMed] [Google Scholar]
- 20.Murphy RM, Zemlan FP. Selective serotonin 1A/1B agonists differentially affect spinal nociceptive reflexes. Neuropharmacology. 1990;29:463–468. doi: 10.1016/0028-3908(90)90168-q. [DOI] [PubMed] [Google Scholar]
- 21.Sufka KJ, Schomburg FM, Giordano J. Receptor mediation of 5-HT-induced inflammation and nociception in rats. Pharmacol Biochem Behav. 1992;41:53–56. doi: 10.1016/0091-3057(92)90058-n. [DOI] [PubMed] [Google Scholar]
- 22.Hunskaar S, Fasmer OB, Broch OJ, Hole K. Involvement of central serotonergic pathways in nefopam-induced antinociception. Eur J Pharmacol. 1987;138:77–82. doi: 10.1016/0014-2999(87)90339-6. [DOI] [PubMed] [Google Scholar]
- 23.Minville V, Fourcade O, Mazoit JX, Girolami JP, Tack I. Ondansetron does not block paracetamol-induced analgesia in a mouse model of fracture pain. Br J Anaesth. 2011;106:112–118. doi: 10.1093/bja/aeq277. [DOI] [PubMed] [Google Scholar]
- 24.Girard P, Coppe MC, Verniers D, Pansart Y, Gillardin JM. Role of catecholamines and serotonin receptor subtypes in nefopam-induced antinociception. Pharmacol Res. 2006;54:195–202. doi: 10.1016/j.phrs.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott JA, Wood M, Flood P. The pronociceptive effect of ondansetron in the setting of P-glycoprotein inhibition. Anesth Analg. 2006;103:742–746. doi: 10.1213/01.ane.0000228861.80314.22. [DOI] [PubMed] [Google Scholar]
- 27.Alghanem SM, Massad IM, Rashed EM, Abu-Ali HM, Daradkeh SS. Optimization of anesthesia antiemetic measures versus combination therapy using dexamethasone or ondansetron for the prevention of postoperative nausea and vomiting. Surg Endosc. 2010;24:353–358. doi: 10.1007/s00464-009-0567-3. [DOI] [PubMed] [Google Scholar]