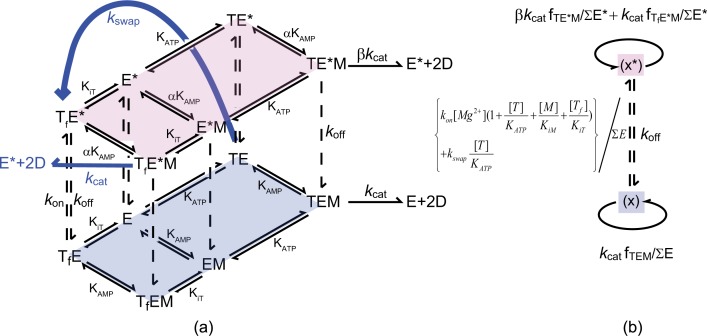
FIGURE 1.
a, the proposed Mg2+-activation model. The forward AK reactions of the unactivated enzyme (E) and its Mg2+ activated counterpart (E*) each follow a random bi-bi model, illustrated by the diamond shapes and assumed to be in rapid equilibrium. The conversion between E and E* (vertical dashed lines) is treated with the steady-state approximation. In the activated form (the upper tier), the dissociation constants KAMP and the catalytic rate kcat are increased by a factor of α and β, respectively (E = AK enzyme, E* = AK·Mg2+, T = ATP·Mg2+, M = AMP, and D = ADP. Tf denotes ATP without Mg2+that has been bound to the enzyme). The blue arrows denote the second order correction to our proposed model. b, a reduction of the model in a according to the hybrid rapid equilibrium and steady-state assumption used to model the kinetic data.