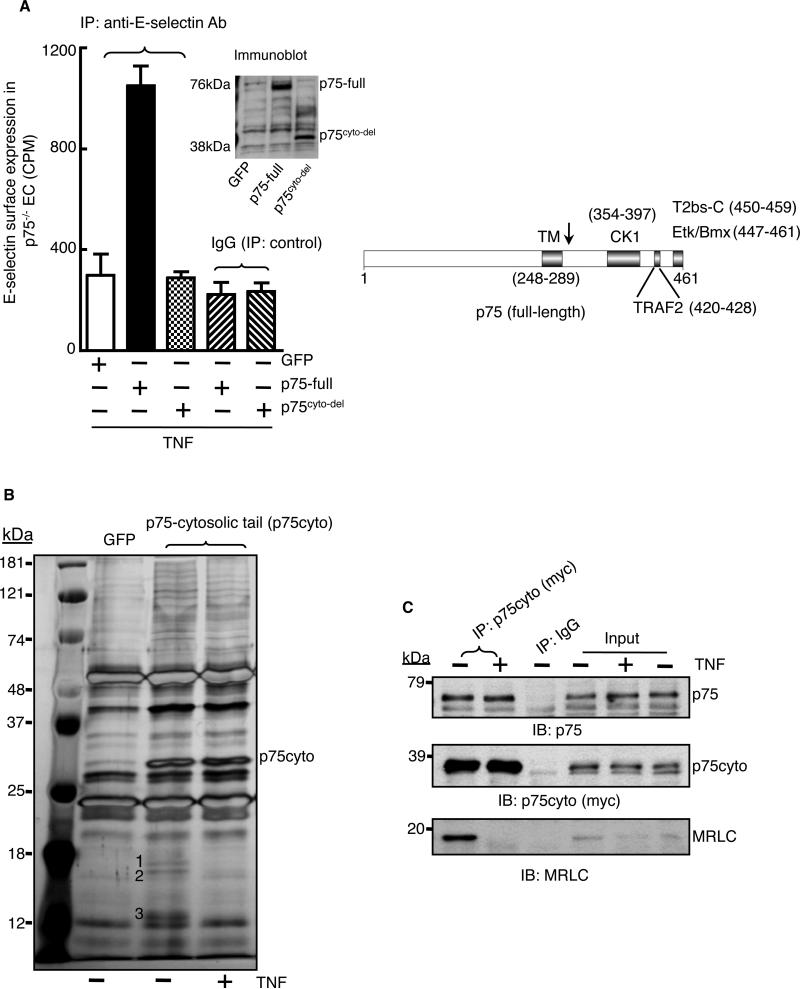
Fig. 1. Nonmuscle myosin II is a p75-associated protein.
(A) Full-length p75 (p75-full) or a mutant p75 protein from which the cytosolic tail was deleted (p75cyto-del) were expressed in endothelial cells isolated from p75-null mice. Cells were treated with TNF-α (2 ng/ml) for 5 hours, and the cell-surface abundance of E-selectin was measured by immunoprecipitation (IP) with a biotin-conjugated anti-E-selectin antibody followed by 125I-labeled streptavidin. GFP expressing cells served as a negative control. Experiments were performed in triplicate with independent endothelial cell isolates and data are expressed as means ± SD. The right panel shows a scheme of the known protein-binding regions in p75 (13, 59, 60). The arrow denotes the point of the truncation in the p75cyto-del mutant protein. (B) The Myc-tagged cytosolic tail of p75 (p75cyto) was immunoprecipitated from control or TNF-α-stimulated (2 ng/ml, 5 min) human endothelial cells with anti-myc antibody, and immunoprecipitates were size-fractionated by SDS-PAGE and then silver-stained. Bands labeled 1 to 3 were excised and analyzed by mass spectrometry and identified as MRLC (bands 1 and 2) and MELC (band 3). (C) The p75cyto protein from untreated or TNF-α-treated human endothelial cells was immuoprecipated with an anti-myc antibody, samples were size-fractionated by SDS-PAGE, and examined by Western blotting (IB) with antibodies specific for p75 and MRLC. Inputs: 5% of the total lysates. Data in (C) are representative of three independent experiments.