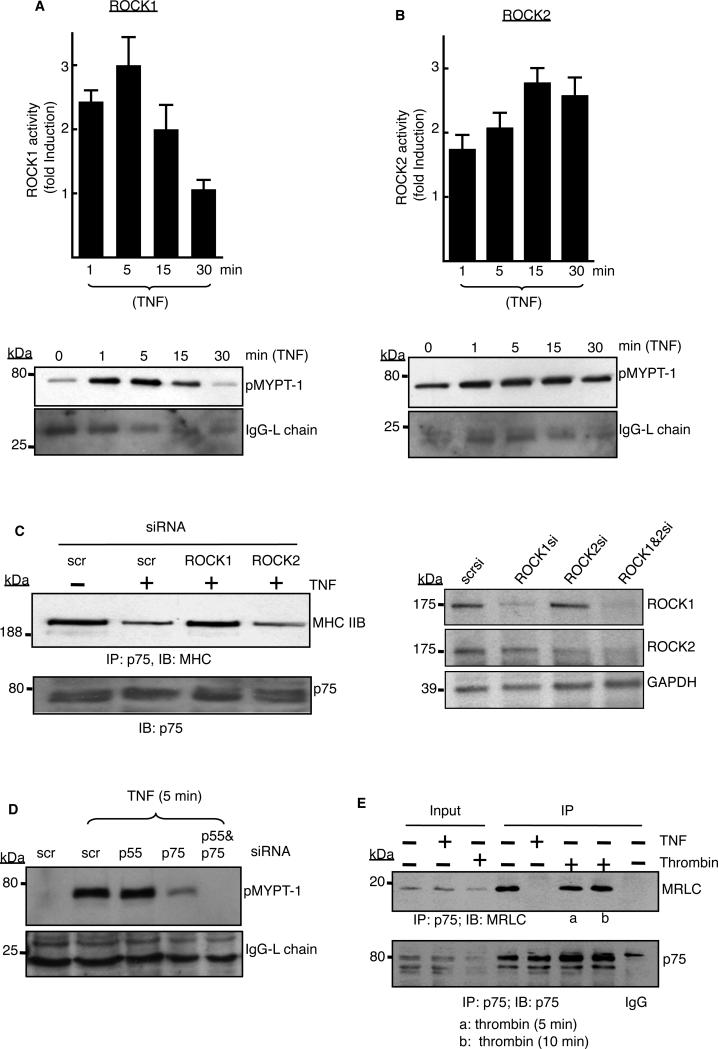
Fig. 4. TNF-α–activated ROCK1 is necessary to release myosin from p75.
(A and B) Endothelial cells were stimulated with TNF-α (2 ng/ml) for the indicated times, ROCK1 or ROCK2 were immunoprecipitated with isoform-specific antibodies, and assays with the precipitated enzymes were performed as described in the Materials and Methods. The extent of phosphorylation of the purified ROCK substrate MYPT-1 (pMYPT-1) was determined by Western blotting analysis. The relative activities of (A) ROCK1 and (B) ROCK2 are shown as the fold-increase in activity with respect to that of untreated cells (defined as one). ROCK activity assays were performed with immunoprecipitates from three independent experiments, and data are expressed as means ± SD. Western blots below the graphs are from representative experiments. The IgG light chain was used as a loading control. (C) Cells transfected with the indicated siRNAs were left untreated or were treated with TNF-α (2 ng/ml) for 5 min before being subjected to immunoprecipitation with antibody against p75, and samples were analyzed by Western blotting with an antibody specific for MHC IIB. Right panel shows Western blotting analysis of the extent of depletion of ROCK1 and ROCK2 by their respective siRNAs. Data are representative of three independent experiments. (D) Cells transfected with the indicated siRNAs were stimulated with TNF-α (2 ng/ml) for 5 min before being subjected to immunoprecipitation with ROCK1-specific antibody. ROCK1 activity was then determined as described earlier. Data are representative of three independent experiments. (E) Cells treated with TNF-α (2 ng/ml, 5 min) or thrombin (5 U/ml) were subjected to immunoprecipitation with antibody against p75 and Western blotting analysis with an MRLC-specific antibody. 5% of the total lysates were used as the inputs. Data are representative of two independent experiments.