Fig. (5).
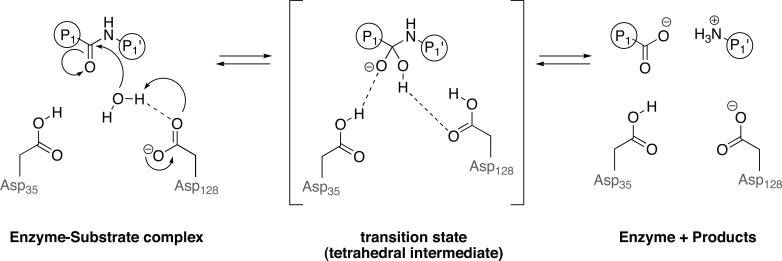
Catalytic mechanism of aspartic peptidase proposed by Nguyen and colleagues 2008 [52]. The water molecule is partly activated by an aspartate and makes a nucleophilic attack at a specific carbonyl carbon in the substrate. The carbonyl oxygen captures a proton from another aspartic acid in the active site, resulting in a tetrahedral intermediate (transition state). Restabilizing from the transition state, the amino moiety from the substrate becomes a better leaving group, and the substrate is cleaved. Dashed lines indicate hydrogen bonds.