Fig 3.
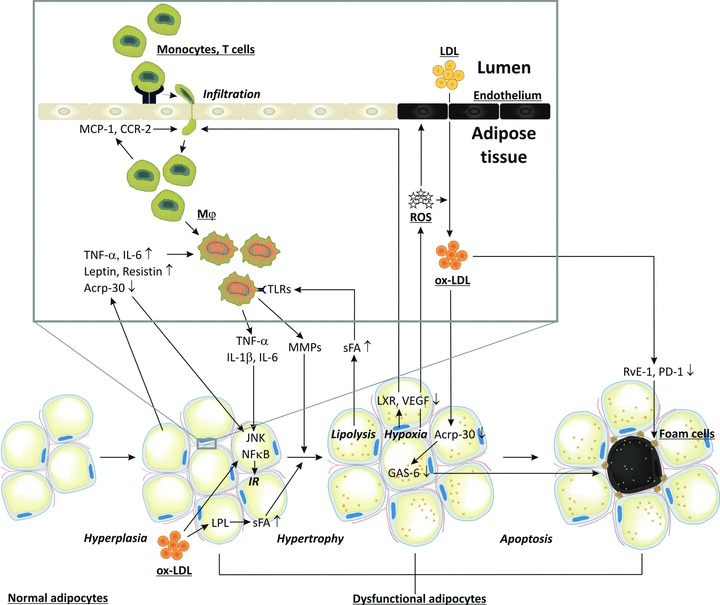
Macrophage infiltration in adipose tissues, oxidative stress and IR. Circulating monocytes adhere to activated endothelial cells. Activated CD8+ T cells and chemokines induce monocyte migration into adipose tissues where they differentiate into macrophages. Interaction of saturated fatty acids with TLRs leads to secretion of inflammatory cytokines/chemokines (IL-6 and TNF-α). Together with the adipocytokines leptin and resistin, they impair c-jun N-terminal kinase and NF-κB signalling, resulting in IR and reduced adiponectin (Acrp-30) secretion, and thereby loss of adipocyte maturation. Together they also induce adipocyte proliferation. Infiltration of inflammatory cells is associated with ROS and ox-LDL production, endothelial cell apoptosis, impaired LXR and VEGF signalling, and decreased blood flow leading to hypoxia and increased oxidative stress. Ox-LDL can further induce adipose tissue hyperplasia, and by inducing lipoprotein lipase it enhances lipid accumulation resulting in adipose tissue hypertrophy. The latter is also facilitated by MMPs secreted by macrophages. Hypoxia and increased oxidative stress induces apoptosis of adipocytes. Increased apoptosis also results from reduced Acrp-30 secretion and growth arrest specific 6 mediated survival pathway. Apoptotic adipocytes attract macrophages which normally remove apoptotic adipocytes. However, ox-LDL impairs phagocytosis of dead adipocytes by inhibiting resolvin E1 and protectin D1 production.
