Fig 1.
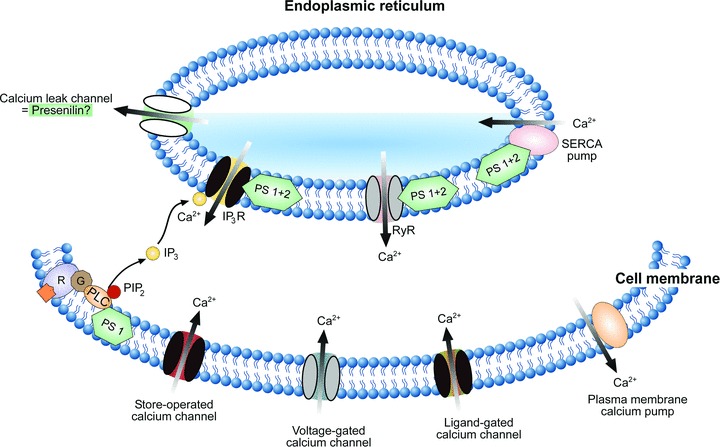
Presenilin-mediated regulation of the intracellular Ca2+ homeostasis. A scheme illustrating the mechanisms underlying intracellular regulation of [Ca2+]i. Ca2+ ions enter the cytosol through ligand-gated, voltage-gated or store-operated Ca2+ channels in the cell membrane. In addition, they are released from the intracellular Ca2+ stores of the ER via IP3 receptor channels (IP3R) or ryanodine receptor-channels (RyR). IP3 is produced from phosphatidylinositol-4,5-bisphosphate (PIP2) by PLC in response to the activation of metabotropic receptors (R). Intracellular Ca2+ levels (30–100 nM at rest, [130]) are controlled by plasma membrane Ca2+ pumps and by SERCAs. Presenilins are located both within the plasma membrane and the ER membrane and interact with many important elements of the Ca2+ signalling cascade (as indicated). Familiar AD mutations in presenilins (i) potentiate PLC activity, (ii) increase Ca2+ release through both IP3 and ryanodine receptors and (iii) modulate activity of SERCA pumps. In addition, presenilins may function as Ca2+ leak channels of the ER (indicated). AD-related mutations in presenilins have been shown to render these leak channels non-functional, thereby causing an overload of the ER Ca2+ stores.
