Abstract
Hydrogen peroxide (H2O2) is involved in intestinal motility through changes of smooth muscle activity. However, there is no report as to the modulatory effects of H2O2 on interstitial cells of Cajal (ICC). We investigated the H2O2 effects and signal transductions to determine whether the intestinal motility can be modulated through ICC. We performed whole-cell patch clamp in cultured ICC from murine intestine and molecular analyses. H2O2 hyperpolarized the membrane and inhibited pacemaker currents. These effects were inhibited by glibenclamide, an inhibitor of ATP-sensitive K+ (KATP) channels. The free-radical scavenger catalase inhibited the H2O2-induced effects. MAFP and AACOCF3 (a cytosolic phospholipase A2 inhibitors) or SC-560 and NS-398 (a selective COX-1 and 2 inhibitor) or AH6809 (an EP2 receptor antagonist) inhibited the H2O2-induced effects. PD98059 (a mitogen activated/ERK-activating protein kinase inhibitor) inhibited the H2O2-induced effects, though SB-203580 (a p38 MAPK inhibitor) or a JNK inhibitor did not affect. H2O2-induced effects could not be inhibited by LY-294002 (an inhibitor of PI3-kinases), calphostin C (a protein kinase C inhibitor) or SQ-22536 (an adenylate cyclase inhibitor). Adenoviral infection analysis revealed H2O2 stimulated tyrosine kinase activity and AG 1478 (an antagonist of epidermal growth factor receptor tyrosine kinase) inhibited the H2O2-induced effects. These results suggest H2O2 can modulate ICC pacemaker activity and this occur by the activation of KATP channels through PGE2 production via receptor tyrosine kinase-dependent MAP kinase activation.
Keywords: hydrogen peroxide, interstitial cells of Cajal, pacemaker currents, cyclooxygenase, receptor tyrosine kinase, MAP kinase
Introduction
Intestinal inflammation either in human beings or in experimental animal models is associated with altered gastrointestinal motility [1–3]. The mechanisms of altered motility have been associated with morphological and functional changes in smooth muscle and enteric nerves [4–6]. Large numbers of polymorphonuclear leucocytes that generate reactive oxygen species (ROS) such as H2O2 are found in the mucosa and submucosa of the inflamed bowel disease, suggesting that H2O2 plays an integral role in the inflammatory process [7]. Several in vitro studies have reported that exposure to H2O2 alters gastrointestinal smooth muscle contractility. For example, H2O2 decreases sigmoid smooth muscle contractility in ulcerative colitis patients and the radical scavenger catalase prevents ulcerative colitis-induced reduction of muscle contractions [8, 9]. Exposure to H2O2 reduces the lower esophageal sphincter tone in human esophagitis and treatment with catalase can restore the lower esophageal tone to normal [10]. From above findings, it is suggested that H2O2 may be an important mediator causing dysmotility in intestinal inflammation.
The gastrointestinal smooth muscles show spontaneous mechanical contractions. These contractions are mediated by the generation of periodic membrane depolarization (slow waves). It is well known that interstitial cells of Cajal (ICC) are pacemaker cells that generate slow waves, which are initiated by spontaneous inward currents (pacemaker currents) [11–13], even if some reports suggested that gastrointestinal pacing was possible without ICC and other pacemaker cells may be involved in pacing activity [14, 15]. ICC are coupled to each other and to smooth muscle cells via gap junctions. ICC also express various receptors for receiving inhibitory and excitatory signals from the enteric nervous systems [16, 17]. Acetic acid-induced inflammation reduces the membrane potential and reduces the amplitude and duration of slow waves in colonic circular muscle cells, suggesting that ICC may involve in motility changes in the inflammatory process [18].
Despite the observation that H2O2 is involved in intestinal motility through the changes of smooth muscle contractility, ion channel activity and enteric neuronal mechanisms, there are no reports describing the modulatory effects of H2O2 on pacemaker activities of ICC. In the present study, we investigated the effects of H2O2 on pacemaker currents and signal transductions to determine whether the intestinal motility can be modulated by ROS through ICC in the murine intestine.
Materials and methods
Preparation of cells and tissues
Balb/C mice (8- to 13-day old) of either sex were anaesthetized with ether and killed by cervical dislocation. The small intestines from 1 cm below the pyloric ring to the cecum were removed and opened along the mesenteric border. The luminal contents were washed away with Krebs-Ringer bicarbonate solution. The tissues were pinned to the base of a Sylgard dish and the mucosa was removed by sharp dissection. Small stripes of intestinal muscle were equilibrated in Ca2+-free Hank’s solution for 30 min and the cells were dispersed with an enzyme solution containing collagenase (Worthington Biochemical Co, Lakewood, NJ, USA), 1.3 mg/ml, bovine serum albumin (Sigma Chemical Co., St. Louis, MO, USA), 2 mg/ml, trypsin inhibitor (Sigma), 2 mg/ml and ATP, 0.27 mg/ml. Cells were plated onto sterile glass coverslips coated with murine collagen (2.5 μg/ml, Falcon/BD) in 35-mm culture dishes. The cells were then cultured at 37°C in a 95% O2–5% CO2 incubator in SMGM (smooth muscle growth medium, Clonetics Corp., San Diego, CA, USA) supplemented with 2% antibiotics/antimycotics (Gibco, Grand Island, NY, USA) and 5 ng/ml murine stem cell factor (SCF, Sigma).
Patch-clamp experiments
Cultures of cells contained single cells and networks of cells that had gross morphological properties similar to ICC in situ, including fusiform cell bodies, large, prominent nuclei with little perinuclear cytoplasm and multiple, thin processes extending from the nuclear region that were often interconnected with processes of neighbouring cells [19]. Recordings were made from ICC with the patch-clamp technique as soon as the network-like structures. Recordings were made from cells within networks that had morphologies similar to the cells that were immunopositive for c-Kit.
The whole-cell configuration of the patch-clamp technique was used to record membrane currents (voltage clamp) and membrane potentials (current clamp) from cultured ICC. Currents or potentials were amplified by use of an Axopatch 1-D (Axon Instruments, Foster City, CA, USA). Command pulse was applied using an IBM-compatible personal computer and pClamp software (version 6.1; Axon Instruments). The data were filtered at 5 kHz and displayed on a computer monitor, and a pen recorder (Gould 2200, Gould, Valley view, OH, USA). All experiments were performed at 30°C.
Results were analysed using pClamp and Graph Pad Prizem (version 2.01) software.
Protein tyrosine kinase assays using adenoviral vectors
Adenoviral vectors encoding enhanced green fluorescent fusion proteins were generated by the two-plasmid rescue system. The following scheme shows a typical protocol for the adenoviral vector assay in live cells using adenoviral vector transduction.
Day 1: Seed appropriate ICC in culture medium with the adenoviral vector at a predetermined multiplicity of infection (MOI) and incubate for 24 hrs
Day 2: H2O2 compounds are added to the incubated samples and the samples are allowed to incubate at 37°C. After incubation, the nuclei of ICC are stained using 2.5 μM Hoechst stain.
Image acquisition (EGFP redistribution assays) was performed on a confocal microscope (×100; fluoviews 300, Olympus) using 360/40 nm (HoechstTM) and 475/20 nm (EGFP) excitation filters and a 535/20 nm emission filter.
Solutions and drugs
The cells were bathed in a solution containing 5 mm KCl, 135 mm NaCl, 2 mm CaCl2, 10 mm glucose, 1.2 mm MgCl2 and 10 mm HEPES, adjusted to pH 7.2 with Tris. The pipette solution contained 140 mm KCl, 5 mm MgCl2, 2.7 mm K2ATP, 0.1 mm Na2GTP, 2.5 mm creatine phosphate disodium, 5 mm HEPES, 0.1 mm EGTA, adjusted to pH 7.2 with Tris.
The drugs used were: hydrogen peroxide, catalase, SC560, AACOCF3, AH6809, glibenclamide, PD98059, SB203580, SQ-22536, JNK inhibitor, NS398, MAFP, LY-294002. PD98059 and JNK inhibitor were purchased from Calbiochem Co., and the other compounds were purchased from the Sigma Chemical Co. (San Diego, CA, USA).
Statistical analysis
Data are expressed as the means ± standard errors. Differences in the data were evaluated by the Student’s t-test. A P values less than 0.05 were taken as a statistically significant difference.
The n values reported in the text refer to the number of cells used in the patch-clamp experiments.
Results
Effects of H2O2 on the pacemaker activity in ICC
To determine whether H2O2 have function on pacemaker activities of ICC, we recorded the pacemaker potential in a current clamp and pacemaker currents in a voltage clamp. Under the current clamp mode, H2O2 (1 mM) produced membrane hyperpolarization and decreased the amplitude of the pacemaker potential (Fig. 1A). Under control conditions at I= 0, the resting membrane potential was −52 ± 4.8 mV, and the amplitude of the pacemaker potential was 39.6 ± 5 mV. In the presence of H2O2, the membrane was hyperpolarized to −76.3 ± 8.0 mV and the amplitude of the pacemaker potentials decreased to 7.6 ± 3.9 mV (n= 6, data not shown). Under a voltage clamp at a holding potential of −70 mV, the ICC generated spontaneous inward currents. The mean frequency of these pacemaker currents was 13.9 ± 1.4 cycles/min and their mean amplitude and mean resting current levels were −416 ± 42 pA and −26 ± 7 pA, respectively (n= 7). The addition of 10 μM H2O2 slightly reduced the amplitude and frequency of pacemaker currents and slightly increased resting currents in the outward direction (Fig. 1B). In the presence of 100 μM and 1 mM H2O2, the pacemaker currents were largely inhibited and the resting currents were increased in the outward direction (Fig. 1C and D). The inhibitory frequencies and amplitudes with H2O2 treatment were 5.1 ± 1.8 cycles/min and −90.9 ± 16 pA at a concentration of 100 μM H2O2 and 2.8 ± 1.4 cycles/min and −24 ± 19 pA at a concentration of 1 mM H2O2, respectively. The resting current levels were 23 ± 5.9 pA at a concentration of 100 μM H2O2 and 24.5 ± 7.2 pA at a concentration of 1 mM H2O2 (n= 7) (Fig. 1E–G). Next, to determine whether H2O2 affects KATP channels in ICC, we used the KATP channels inhibitor glibenclamide, after or before H2O2 treatment. The H2O2-induced effects on pacemaker currents were inhibited by co- or pretreatment with glibenclamide (Fig. 2A and E), indicating that H2O2 may activate KATP channels in ICC. The results of glibenclamide treatment on the H2O2-induced effects on pacemaker currents are summarized in Figure 2B–D.
Fig 1.
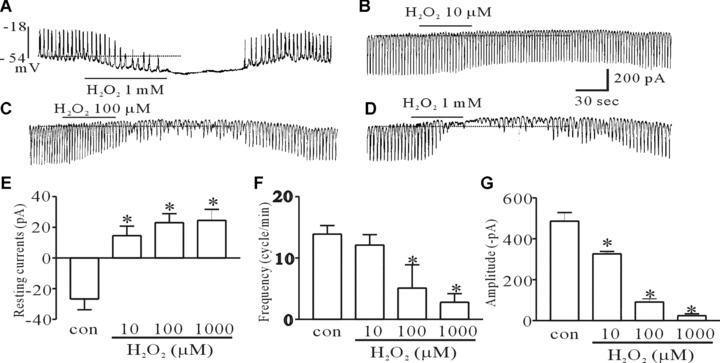
The effect of H2O2 on pacemaker potentials and currents in cultured ICC of the murine intestine. (A) Pacemaker potentials of ICC exposed to 1-mM H2O2 in the current clamping mode (I = 0). (B, C and D) Pacemaker currents of ICC exposed to H2O2 (10, 100 μM and 1 mM) at a holding potential of −70 mV. (E, F and G) A summarized bar graph showing the H2O2-induced effects on pacemaker currents of ICC. The bars represent mean ± S.E. values (n= 7/group). Asterisks indicate significantly different from the controls (P < 0.05), and the dotted lines indicate zero current levels.
Fig 2.
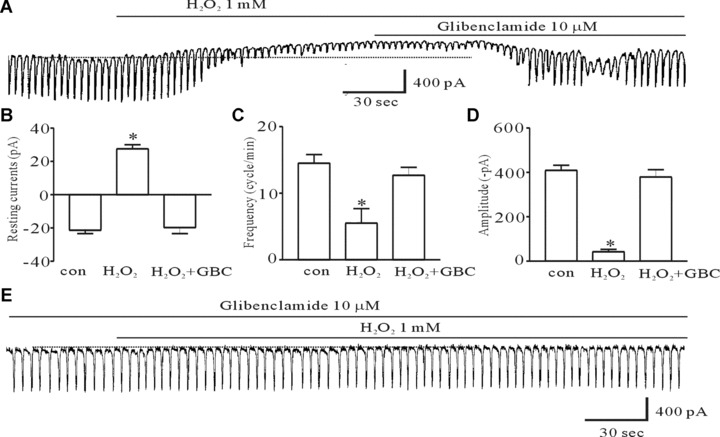
The effect of glibenclamide, an inhibitor of ATP-sensitive K+ channels, on H2O2-induced action on pacemaker currents of ICC from the murine intestine. (A) Pacemaker currents exposed to 1 mM H2O2 at a holding potential of −70 mV. The H2O2-induced effects were reversed by adding 10 μM glibenclamide. (B, C, and D) The inhibitory response to glibenclamide on the H2O2-induced action on pacemaker currents. (E) The effect of 1-mM H2O2 on pacemaker currents after pretreating cells with 10 μM glibenclamide. The bars represent mean ± S.E. values (n= 6). Asterisks indicate significantly different from the controls (P < 0.05), and the dotted lines indicate zero current levels. glibenclamide, GBC.
Effects of catalase and phospolipase A2 inhibitors on the H2O2-induced inhibition of pacemaker currents
To evaluate whether the H2O2-induced inhibition of pacemaker currents was mediated via a ROS and PLA2 signal pathway, we treated the ICC with catalase (3000 unit/ml), MAFP (10 μM) and AACOCF3 (10 μM) before exposure to H2O2. Catalase inhibited the H2O2-induced effects (Fig. 3A) indicating that the H2O2-induced effects may be an ROS-dependent mechanism. Figure 3B and C shows that the 1 mM H2O2-induced effects were significantly inhibited by treatment with MAFP and AACOCF3. As shown in Figure 3C–E, the values of the frequency, amplitude and resting currents by H2O2 in the presence of catalase, MAFP and AACOCF3 were significantly different from those obtained in the absence of catalase, MAFP and AACOCF3 (n= 5).
Fig 3.
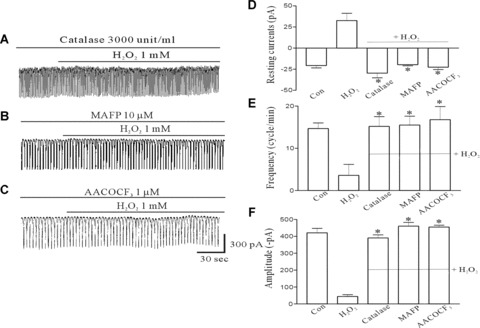
The effect of catalase, a radical scavenger, MAFP, a non-specific phospholipase A2 inhibitor, and AACOCF3, a cytosolic phospholipase A2 inhibitor on the H2O2-induced action on pacemaker currents of ICC from the murine intestine. (A) The effect of 1 mM H2O2 on pacemaker currents after pretreating cells with catalase (3000 units/ml). (B) Pacemaker currents exposed to 1 mM H2O2 after pretreating cells with 10 μM MAFP at a holding potential of −70 mV. (C) The effect of 1 mM H2O2 on pacemaker currents after pretreating cells with 10 μM AACOCF3. (D, E and F) The inhibitory response to catalase, MAFP or AACOCF3 on the H2O2-induced effects on pacemaker currents. The bars represent mean ± S.E. values (n= 6). Asterisks indicate significantly different from H2O2 alone (P < 0.05).
Involvement of cyclooxygenase and the prostaglandin E2 receptor in the H2O2-induced inhibition of pacemaker currents
Since activation of the PLA2 pathway would stimulate the cyclooxygenase (COX) and prostaglandins synthesis, we examined the role of this system in mediating the H2O2-induced effects using SC-560 (10 μM), a specific COX-1 inhibitor, or NS-398 (10 μM), a specific COX-2 inhibitor. ICC were pretreated with SC-560 (10 μM) or NS-398 (10 μM) prior to the treatment with H2O2. We found that the H2O2-induced effects was inhibited by pretreatment with SC-560 or NS-398 (n= 7, Fig. 4A and B). Our previous report suggested that PGE2-activated KATP channels through PGE-EP2 receptor activation in ICC [20]. Therefore, we tested AH6809 (10 μM), a PGE2-EP2 receptor antagonist and found the H2O2-induced effects were inhibited by pretreatment with AH6809 (Fig. 5A). Furthermore, AH6809 inhibited the PGE2-induced effects (Fig. 5B). The values of the frequency, amplitude and resting currents induced by H2O2 in the presence of AH6809 were significantly different from those obtained in the absence of AH-6809 (n= 6, Fig. 5C–E). These results suggest that PGE2 may be involved in the H2O2-induced inhibition of pacemaker currents.
Fig 4.
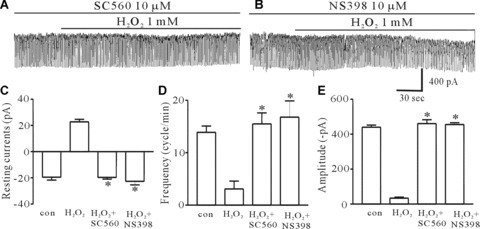
The effect of SC-560, a specific cyclooxgenase-1 inhibitor, and NS-398, a specific cyclooxygenase-2 inhibitor, on the H2O2-induced effects on pacemaker currents of ICC from the murine intestine. (A) Pacemaker currents exposed to 1 mM H2O2 after pretreating cells with 10 μM SC-560 at a holding potential of −70 mV. (B) The effect of 1 mM H2O2 on pacemaker currents after pretreating cells with 10 μM NS-398. (C, D and E) The inhibitory response to SC-560 or NS-398 on the H2O2-induced action on pacemaker currents. The bars represent mean ± S.E. values (n= 7). Asterisks indicate significantly different from H2O2 alone (P < 0.05).
Fig 5.

The effect of AH6809, a PGE2-EP2 receptor antagonist, on the H2O2-induced effects on pacemaker currents of ICC from the murine intestine. (A) The effect of 1 mM H2O2 on pacemaker currents after pretreating cells with 10 μM AH6809. (B) The effect of 1 μM PGE2 on pacemaker currents after pretreating cells with 10 μM AH6809. (C, D and E) The inhibitory response to AH6809 on the H2O2-induced effects on pacemaker currents. The bars represent mean ± S.E. values (n= 6). Asterisks indicate significantly different from H2O2 alone (P < 0.05).
Involvement of mitogen-activated protein kinases (MAPKs) in the H2O2-induced inhibition of pacemaker currents
Since many reports suggested H2O2 activate MAPKs in many cell types, we investigated whether MAPKs are involved in the H2O2-induced effects using PD98059, a p44/42 MAPK inhibitor, or SB203580, a p38 MAPK inhibitor, or a JNK (c-jun NH2-terminal kinse) inhibitor. Figure 6A shows that PD98059 (10 μM) prevented the H2O2-induced effects. However, SB203580 and JNK inhibitor did not affect the H2O2-induced effects (Fig. 6B and C). The values of the frequency, amplitude and resting currents by H2O2 in the presence of PD98059 were significantly different from those obtained in the absence of PD98059 (n= 6, Fig. 6E–G). In addition, PD98059 inhibited the PGE2-induced effects; supporting the role of PGE2 as a mediator of H2O2 (Fig. 6D).
Fig 6.
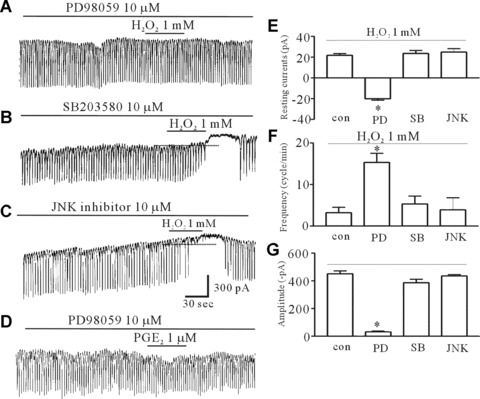
The effect of PD98059, SB203580 and the JNK inhibitor on the H2O2-induced effects on pacemaker currents of ICC from the murine intestine. (A) The effect of 1 mM H2O2 on pacemaker currents after pretreating cells with 10 μM PD98059, a p44/42 MAPK inhibitor, for 15 min. (B) The effect of 1 mM H2O2 on pacemaker currents after pretreating cells with 10 μM SB203580, a p38 MAPK inhibitor, for 15 min. (C) The effect of 1 mM H2O2 on pacemaker currents after pretreating cells with 10 μM JNK inhibitor, c-jun NH2-terminal kinase inhibitor, for 15 min. (D) The effect of 1 μM PGE2 on pacemaker currents after pretreating cells with 10 μM PD98059 for 15 min. (E, F, and G) The inhibitory response to PD98059 on the H2O2-induced action on pacemaker currents. The bars represent mean ± S.E. values (n= 6/group). Asterisks indicate significantly different from H2O2 alone (P < 0.05), and the dotted lines indicate zero current levels. PD98059, PD; SB203580, SB; JNK inhibitor, JNK.
Effects of the adenylate cyclase inhibitor, PI3-kinase inhibitor and protein kinase C inhibitor in the H2O2-induced inhibition of pacemaker currents
To determine an upstream regulator of p44/42 activation, ICC were pretreated with either 10 μM SQ-22536 (an adenylate cyclase inhibitor), 10 μM LY-294002 (a PI3-kinase inhibitor) and 0.1 μM calphostin C (a PKC inhibitor). We found that all inhibitors did not affect the H2O2-induced effects (n= 5: bar graph not shown), indicating that the H2O2-induced effects in ICC are part of a cAMP-, PI3-kinase- and PKC-independent pathway (Fig. 7A–C).
Fig 7.
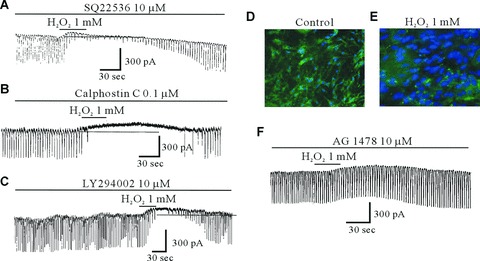
The effect of SQ-22536, calphostin C, LY-294002 and AG1478 on the H2O2-induced effects on pacemaker currents of ICC and images from the ICC expressing the tyrosine kinase fusion protein, delivered by an adenoviral vector from the murine intestine. (A) The effect of 1 mM H2O2 on pacemaker currents after pretreating cells with 10 μM SQ22536, an adenylate cyclase inhibitor, for 10 min. (B) The effect of 1 mM H2O2 on pacemaker currents after pretreating cells with 0.1 μM calphostin C, protein kinase C inhibitor, for 10 min. (C) The effect of 1 mM H2O2 on pacemaker currents after pretreating cells with 10 μM LY-294002, PI3-kinase inhibitor, for 15 min. (D) In resting cells: a punctuate pattern of fluorescence is observed. (E) When ICC are treated with 1 mM H2O2 for 30 min., the ICC becomes diffuse. (F) The effect of 1 mM H2O2 on pacemaker currents after pretreating cells with 10 μM AG1478, a potent and selective inhibitor of epidermal growth factor receptor, for 15 min. (G) The effect of 1 μM PGE2 on pacemaker currents after pretreating cells with 10 μM AG1478. The dotted lines indicate zero current levels.
Involvement of EGFR (epidermal growth factor receptor) tyrosine kinase in the H2O2-induced inhibition of pacemaker currents
To evaluate the role of tyrosine kinase in the H2O2-induced effects, ICC were infected with adenovirus that was constructed containing a tyrosine kinase gene. In Figure 7D, we could see the tyrosine kinase domain was located predominantly in the nucleus of ICC. When ICC were treated with H2O2 (1 mM) for 30 min, there was a translocation of the tyrosine kinase from nucleus to the cytosol (Fig. 7E). This finding indicates that H2O2 stimulates the tyrosine kinase activity in ICC. Furthermore, to determine whether the H2O2-induced inhibition of pacemaker currents is mediated by EGFR tyrosine kinase, we treated the cells with AG 1478, a potent and selective inhibitor of EGFR tyrosine kinase. AG 1478 (10 μM) inhibited the H2O2-induced effects (Fig. 7F). Also, we found that AG 1478 inhibited the PGE2-induced inhibition of pacemaker currents (Fig. 7G). This finding suggests that the H2O2-induced effect is dependent on EGFR tyrosine kinase activation through PGE2.
Discussion
In the present study, we first demonstrated that H2O2 induces hyperpolarization of the membrane and inhibits pacemaker currents in intestinal ICC. These effects are mediated through activation of KATP channels by COX-dependent PGE2 production, and receptor tyrosine and MAP kinase are involved in the H2O2-induced process.
K+ channels play an important role in regulating cellular excitability in various cell types. H2O2 hyperpolarizes the resting membrane potential through the activation of several K+ channels in vascular and visceral smooth muscles [21, 22]. The activation of KATP channels would lead to membrane hyperpolarization, which is thought to be an important mechanism for smooth muscle relaxation. We have already functionally reported that ICC have an KATP channels and that the ICC are modulated by bile salts and antidepressants [23, 24]. Deoxycholic acid inhibited pacemaker currents that were blocked by glibenclamide, an inhibitor of the KATP channel, and imipramine blocked the pinacidil (an opener of the KATP channels)-induced inhibition in a similar manner as glibenclamide. KATP channels is comprised of Kir 6.2 with SUR2B in cultured ICC of the mouse [20], indicating these channels may play an important role in the electrical activities of ICC and be a target of endogenous substances and drugs, as in smooth muscle. In the present study, H2O2 hyperpolarized the membrane potential and inhibited pacemaker currents. These effects were inhibited by glibenclamide, which indicates that H2O2 can change pacemaker activities through the activation of KATP channels in ICC.
H2O2 mediates the production of PGs from arachidonic acid via COX enzyme activation in GI tract. For examples, exposure to H2O2 causes damage to the plasma membrane of the gallbladder muscle and contraction through the generation of PGE2 by the cPLA2-cyclooxygenase pathway [25]. H2O2 reduces the lower esophageal sphincter (LES) tone in human esophagitis and the cat LES tone by increasing the synthesis of COX-2 and PGE2[10, 26]. And we reported that deoxycholic acid activated COX-2-dependent PGE2 production [23]. The biological effects of PGE2 are mediated via four different receptor subtypes (EP1, -2, -3 and -4) [27]. We found that PGE2 action on pacemaker currents in ICC was mediated by EP2 receptors and the expression of the EP2 subtype was only detected [20]. In the present study, H2O2-induced effects were blocked by cPLA2 inhibitors (MAFP and AACOCF3) or COX inhibitors (SC560 and NS-398) as well as by an EP2-receptor antagonist (AH6809). Taken together, these results suggest that the inhibition of pacemaker currents through activation of KATP channels by H2O2 is mediated by cPLA2-COX-dependent PGE2 production.
A remaining issue to consider is the determination of the signal linkage between production of PGE2 by H2O2 and the activation of KATP channels. The MAPKs signaling pathway plays an important role in the mediation of cellular responses including visceral smooth muscle contraction [28]. Three principal MAPKs are expressed in various tissues: p44/42 MAPK, JNK and p38 MAPK [29]. H2O2 and PGE2 activate MAPKs in many cell types [30–32]. In the present study, PD98059, an inhibitor of p44/42 MAPK, inhibited the H2O2- or PGE2-induced inhibition of pacemaker currents suggesting p44/42 MAPK may be involved in the modulation of pacemaker currents by H2O2. It has been reported that H2O2 induced ERK1/2 activation in cultured feline ileal smooth muscle cells and pulmonary arterial smooth muscle cells [32, 33]. In addition, MAPK-ERK is involved in the activation of KATP channel by nitric oxide in neurons [34]. In addition, PGE2 activates ERK1/2 in several cells [35, 36], which supports our results.
cAMP, PI3-kinase, PKC and tyrosine kinase are upstream regulators in MAPKs activation [22, 37]. The cAMP pathway is the major inhibitory mechanism responsible for the relaxation of gastrointestinal smooth muscle [38]. In addition, KATP channels are opened by the cAMP-dependent signal pathway in smooth muscle cells [39]. However, in the current study, treatment with an adenylate cyclase inhibitor had no influence on the H2O2-induced effects. Also, cell permeable 8-bromo-cAMP did not inhibit pacemaker currents [40], indicating cAMP is not involved in the H2O2-induced effects. An antagonist of PI3-kinase, LY-294002 suppresses H2O2-induced contraction in vascular smooth muscle cells [41]. PI3-kinase also has been reported to mediate ERK1/2 activation by insulin and thrombin [42, 43]. PI3 kinase leads to activation of PKC [44]. In addition, H2O2 has been reported to stimulate PKC activities [45]. Also, some reports suggested that spontaneous rhythmic contractions of uterus and intestine were inhibited by a novel and potent inhibitor of c-Kit tyrosine kinase [46, 47]. In this study, LY-294002 or PKC blocker (calphostin C) did not affect H2O2-induced effects. Generally PKC activation produces smooth muscle contraction. Therefore, these results suggest that cAMP, PI3-kinase and PKC are not involved in the H2O2-signal pathways, indicating that other mechanisms may be involved in the activation of KATP channels in intestinal ICC by H2O2.
H2O2 increases tyrosine phosphorylation of proteins in different cell types [32]. Receptor tyrosine kinase, such as platelet-derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR) and insulin-like growth factor (IGFR), have been demonstrated to be targets of ROS [48, 49]. It has been reported that H2O2-induced p44/42 activation was mediated by the receptor tyrosine kinase of EGFR in feline ileal smooth muscle cells and vascular smooth muscle cells [32, 33]. Based on these findings, we examined the activation of EGFR using an EGFR antagonist (AG1478) before exposure to H2O2. We found that EGFR antagonist inhibited the H2O2-induced effects. It has been reported that PGE2 induced EGRF transactivation, leading to the promotion of gastrointestinal cancer cell growth [35]. Furthermore, the PGE2 receptor EP4 agonist promotes hepatocytes proliferation through EGFR and ERK phosphorylation [50]. In the present study, an EGFR receptor tyrosine kinase inhibitor inhibited PGE2-induced effects. Taken together these findings suggest that the activation of EGFR by H2O2 via PGE2 may involve the regulation of pacemaker currents.
In conclusion, we have demonstrated that the inhibition of pacemaker currents by H2O2 in cultured ICC has mediated the activation of COX, with a consequent increase of PGE2 production. And we have showed that during these events, the increasing of PGE2 can influence on KATP channels, the receptor tyrosine kinase of EGFR and also MAPK (p44/42) pathway.
Acknowledgments
This work was supported by a grant from the Clinical Research Center of the Chosun University Hospital (2007) and a grant [2009-0065525] from the Ministry of Science and technology, the Korean government.
References
- 1.Reddy SN, Bazzocchi G, Chan S, et al. Colonic motility and transit in health and ulcerative colitis. Gastroenterology. 1991;101:1289–97. doi: 10.1016/0016-5085(91)90079-z. [DOI] [PubMed] [Google Scholar]
- 2.Vrees MD, Pricolo VE, Potenti FM, et al. Abnormal motility in patients with ulcerative colitis: the role of inflammatory cytokines. Arch Surg. 2002;137:439–45. doi: 10.1001/archsurg.137.4.439. [DOI] [PubMed] [Google Scholar]
- 3.Myers BS, Martin JS, Dempsey DT, et al. Acute experimental colitis decreases colonic circular smooth muscle contractility in rats. Am J Physiol. 1997;273:G928–36. doi: 10.1152/ajpgi.1997.273.4.G928. [DOI] [PubMed] [Google Scholar]
- 4.Goldhill JM, Sanders KM, Sjogren R, et al. Changes in enteric neural regulation of smooth muscle in a rabbit model of small intestinal inflammation. Am J Physiol. 1995;268:G823–30. doi: 10.1152/ajpgi.1995.268.5.G823. [DOI] [PubMed] [Google Scholar]
- 5.Blennerhassett MG, Vignjevic P, Vermillion DL, et al. Inflammation causes hyperplasia and hypertrophy in smooth muscle of rat small intestine. Am J Physiol. 1992;262:G1041–6. doi: 10.1152/ajpgi.1992.262.6.G1041. [DOI] [PubMed] [Google Scholar]
- 6.Ozaki H, Hori M, Kinoshita K, et al. Intestinal dysmotility in inflammatory bowel disease: mechanisms of the reduced activity of smooth muscle contraction. Inflammopharmacology. 2005;13:103–11. doi: 10.1163/156856005774423773. [DOI] [PubMed] [Google Scholar]
- 7.Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859–61. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- 8.Cao W, Vrees MD, Potenti FM, et al. Interleukin 1beta-induced production of H2O2 contributes to reduced sigmoid colonic circular smooth muscle contractility in ulcerative colitis. J Pharmacol Exp Ther. 2004;311:60–70. doi: 10.1124/jpet.104.068023. [DOI] [PubMed] [Google Scholar]
- 9.Cao W, Fiocchi C, Pricolo VE. Production of IL-1beta, hydrogen peroxide, and nitric oxide by colonic mucosa decreases sigmoid smooth muscle contractility in ulcerative colitis. Am J Physiol Cell Physiol. 2005;289:C1408–16. doi: 10.1152/ajpcell.00073.2005. [DOI] [PubMed] [Google Scholar]
- 10.Cheng L, Harnett KM, Cao W, et al. Hydrogen peroxide reduces lower esophageal sphincter tone in human esophagitis. Gastroenterology. 2005;129:1675–85. doi: 10.1053/j.gastro.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Ward SM, Burns AJ, Torihashi S, et al. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–7. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huizinga JD, Thuneberg L, Kluppel M, et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–9. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 13.Thomsen L, Robinson TL, Lee JC, et al. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998;4:848–51. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- 14.Daniel EE, Willis A, Cho WJ, et al. Comparisons of neural and pacing activities in intestinal segment for W/W++ and W/Wv mice. Neurogastroenterol Motil. 2005;17:355–65. doi: 10.1111/j.1365-2982.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 15.Yin J, Hou X, Chen JDZ. Roles of interstitial cells of Cajal in intestinal transit and exogenous electrical pacing. Dig Dis Sci. 2006;51:1818–23. doi: 10.1007/s10620-006-9313-z. [DOI] [PubMed] [Google Scholar]
- 16.Faussone-Pellegrini MS. Relationships between neurokinin receptor-expressing interstitial cells of Cajal and tachykininergic nerves in the gut. J Cell Mol Med. 2006;10:20–32. doi: 10.1111/j.1582-4934.2006.tb00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward SM, Sanders KM, Hirst GD. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol Motil. 2004;16:112–7. doi: 10.1111/j.1743-3150.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 18.Lu G, Qian X, Berezin I, et al. Inflammation modulates in vitro colonic myoelectric and contractile activity and interstitial cells of Cajal. Am J Physiol. 1997;273:G1233–45. doi: 10.1152/ajpgi.1997.273.6.G1233. [DOI] [PubMed] [Google Scholar]
- 19.Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 1998;513:203–13. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi S, Yeum CH, Chang IY, et al. Activating of ATP-dependent K+ channels comprised of Kir 6.2 and SUR 2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine. Cell Physiol Biochem. 2006;18:187–98. doi: 10.1159/000097516. [DOI] [PubMed] [Google Scholar]
- 21.Lu G, Mazet B, Sun C, et al. Inflammatory modulation of calcium-activated potassium channels in canine colonic circular smooth muscle cells. Gastroenterology. 1999;116:884–92. doi: 10.1016/s0016-5085(99)70071-5. [DOI] [PubMed] [Google Scholar]
- 22.Prasad M, Goyal RK. Differential modulation of voltage-dependent K+ currents in colonic smooth muscle by oxidants. Am J Physiol Cell Physiol. 2004;286:C671–82. doi: 10.1152/ajpcell.00137.2003. [DOI] [PubMed] [Google Scholar]
- 23.Jun JY, Choi S, Chang IY, et al. Deoxycholic acid inhibits pacemaker currents by activating ATP-dependent K+ channels through prostaglandin E2 in interstitial cells of Cajal from the murine small intestine. Br J Pharmacol. 2005;144:242–51. doi: 10.1038/sj.bjp.0706074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi S, Park CG, Kim MY, et al. Action of imipramine on activated ATP-sensitive K+ channels in interstitial cells of Cajal from murine small intestine. Life Sci. 2006;78:2322–8. doi: 10.1016/j.lfs.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Xiao ZL, Andrada MJ, Biancani P, et al. Reactive oxygen species (H2O2): effects on the gallbladder muscle of guinea pigs. Am J Physiol Gastrointest Liver Physiol. 2002;282:G300–6. doi: 10.1152/ajpgi.00241.2001. [DOI] [PubMed] [Google Scholar]
- 26.Cao W, Cheng L, Behar J, et al. IL-1 beta signaling in Cat Lower Esophageal Sphincter (LES) Circular Muscle. Am J Physiol Gastrointest Liver Physiol. 2006;291:G672–80. doi: 10.1152/ajpgi.00110.2006. [DOI] [PubMed] [Google Scholar]
- 27.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 28.Cao W, Sohn UD, Bitar KN, et al. MAPK mediates PKC-dependent contraction of cat esophageal and lower esophageal sphincter circular smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;285:G86–95. doi: 10.1152/ajpgi.00156.2002. [DOI] [PubMed] [Google Scholar]
- 29.Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Jin N, Liu Y, et al. Hydrogen peroxide stimulates extracellular signal-regulated protein kinases in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol. 1998;19:324–32. doi: 10.1165/ajrcmb.19.2.3209. [DOI] [PubMed] [Google Scholar]
- 31.Abe MK, Kartha S, Karpova AY, et al. Hydrogen peroxide activates extracellular signal-regulated kinase via protein kinase C, Raf-1, and MEK1. Am J Respir Cell Mol Biol. 1998;18:562–9. doi: 10.1165/ajrcmb.18.4.2958. [DOI] [PubMed] [Google Scholar]
- 32.Tabet F, Schiffrin EL, Touyz RM. Mitogen-activated protein kinase activation by hydrogen peroxide is mediated through tyrosine kinase-dependent, protein kinase C-independent pathways in vascular smooth muscle cells: upregulation in spontaneously hypertensive rats. J Hypertens. 2005;23:2005–12. doi: 10.1097/01.hjh.0000185715.60788.1b. [DOI] [PubMed] [Google Scholar]
- 33.Song HJ, Lee TS, Jeong JH, et al. Hydrogen peroxide-induced extracellular signal-regulated kinase activation in cultured feline ileal smooth muscle cells. J Pharmacol Exp Ther. 2005;312:391–8. doi: 10.1124/jpet.104.074401. [DOI] [PubMed] [Google Scholar]
- 34.Lin YF, Raab-Graham K, Jan YN, et al. NO stimulation of ATP-sensitive potassium channels: involvement of Ras/mitogen-activated protein kinase pathway and contribution to neuroprotection. Proc Natl Acad Sci USA. 2004;101:7799–804. doi: 10.1073/pnas.0402496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pai R, Soreghan B, Szabo IL, et al. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289–93. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 36.Jabbour HN, Boddy SC. Prostaglandin E2 induces proliferation of glandular epithelial cells of the human endometrium via extracellular regulated kinase 1/2-mediated pathway. J Clin Endocrinol Metab. 2003;88:4481–7. doi: 10.1210/jc.2003-030297. [DOI] [PubMed] [Google Scholar]
- 37.Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002;12:258–66. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- 38.Sanders KM. G protein-coupled receptors in gastrointestinal physiology. IV. Neural regulation of gastrointestinal smooth muscle. Am J Physiol. 1998;275:G1–7. doi: 10.1152/ajpgi.1998.275.1.G1. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Bonev AD, Mawe GM, et al. Protein kinase A mediates activation of ATP-sensitive K+ currents by CGRP in gallbladder smooth muscle. Am J Physiol. 1994;267:G494–9. doi: 10.1152/ajpgi.1994.267.3.G494. [DOI] [PubMed] [Google Scholar]
- 40.Jun JY, Choi S, Yeum CH, et al. Noradrenaline inhibits pacemaker currents through stimulation of beta 1-adrenoceptors in cultured interstitial cells of Cajal from murine small intestine. Br J Pharmacol. 2004;141:670–7. doi: 10.1038/sj.bjp.0705665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang ZW, Zheng T, Wang J, et al. Hydrogen peroxide induces contraction and raises [Ca2+]i in canine cerebral arterial smooth muscle: participation of cellular signaling pathways. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:646–53. doi: 10.1007/s002109900128. [DOI] [PubMed] [Google Scholar]
- 42.Cross DA, Alessi DR, Vandenheede JR, et al. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem J. 1994;303:21–6. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawes BE, Luttrell LM, Van Biesen T, et al. Phosphatidylinositol 3-kinase is an early intermediate in the G beta gamma-mediated mitogen-activated protein kinase signaling pathway. J Biol Chem. 1996;271:12133–6. doi: 10.1074/jbc.271.21.12133. [DOI] [PubMed] [Google Scholar]
- 44.Cao W, Sohn UD, Bitar KN, et al. MAPK mediates PKC-dependent contraction of cat esophageal and lower esophageal sphincter circular smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;285:G86–95. doi: 10.1152/ajpgi.00156.2002. [DOI] [PubMed] [Google Scholar]
- 45.Polosukhina D, Singaravelu K, Padanilam BJ. Activation of protein kinase C isozymes protects LLCPK1 cells from H2O2 induced necrotic cell death. Am J Nephrol. 2003;23:380–9. doi: 10.1159/000073984. [DOI] [PubMed] [Google Scholar]
- 46.Shimojima N, Nakaki T, Morikawa Y, et al. Imatinib blocks spontaneous mechanical activities in the adult mouse small intestine: possible inhibition of c-Kit signaling. Pharmacology. 2005;74:95–9. doi: 10.1159/000084021. [DOI] [PubMed] [Google Scholar]
- 47.Popescu LM, Vidulescu C, Curici A, et al. Imatinib inhibits spontaneous rhythmic contractions of human uterus and intestine. Eur J Pharmacol. 2006;546:177–81. doi: 10.1016/j.ejphar.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 48.Hackel PO, Zwick E, Prenzel N, et al. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999;11:184–9. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- 49.Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–83. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 50.Kataoka K, Takikawa Y, Lin SD, et al. Prostaglandin E2 receptor EP4 agonist induces Bcl-xL and independently activates proliferation signals in mouse primary hepatocytes. J Gastroenterol. 2005;40:610–6. doi: 10.1007/s00535-005-1595-y. [DOI] [PubMed] [Google Scholar]


