Table 1.
Catalytic enantioselective allylic alkylations with vinylaluminum reagent 3 promoted by NHC–Cu complex derived from 5.[a]
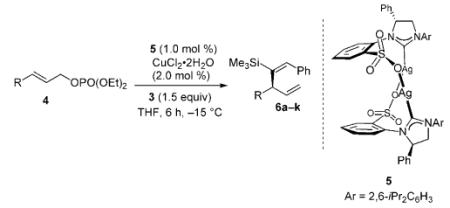
| Entry | Substrate [R] | Yield [%][b] | SN2′:SN2[c] | E:Z[c] | e.r.[d] |
|---|---|---|---|---|---|
| 1 | 4a [Ph] | 93 | >98:2 | >98:2 | 99:1 |
| 2 | 4b [oMeC6H4] | 96 | >98:2 | >98:2 | 99:1 |
| 3 | 4c [oMeOC6H4] | 82 | >98:2 | >98:2 | 98:2 |
| 4 | 4d [oNO2C6H4] | 89 | >98:2 | >98:2 | >99:1 |
| 5 | 4e [mBrC6H4] | 95 | >98:2 | >98:2 | 99:1 |
| 6 | 4 f [pClC6H4] | 94 | >98:2 | >98:2 | 98.5:1.5 |
| 7 | 4g [pNO2C6H4] | 87 | >98:2 | >98:2 | 97.5:2.5 |
| 8 | 4h [pTsOC6H4] | >98 | >98:2 | >98:2 | 99:1 |
| 9 | 4i [pMeC6H4] | 92 | >98:2 | >98:2 | >99:1 |
| 10 | 4j [(CH2)2C6H5] | 88 | >98:2 | >98:2 | 94:6 |
| 11 | 4k [Cy] | 88 | >98:2 | >98:2 | 94:6 |
Reactions were performed under N2 atmosphere; >98% conversion and <2% iBu addition product in all cases (400 MHz 1H NMR analysis of unpurified product mixtures).
Yields of isolated purified products.
Site-selectivities and alkene stereoselectivities established by analysis of 400 MHz 1H NMR spectra of product mixtures prior to purification.
Enantiomer ratios determined by HPLC analysis; see the Supporting Information for details.
