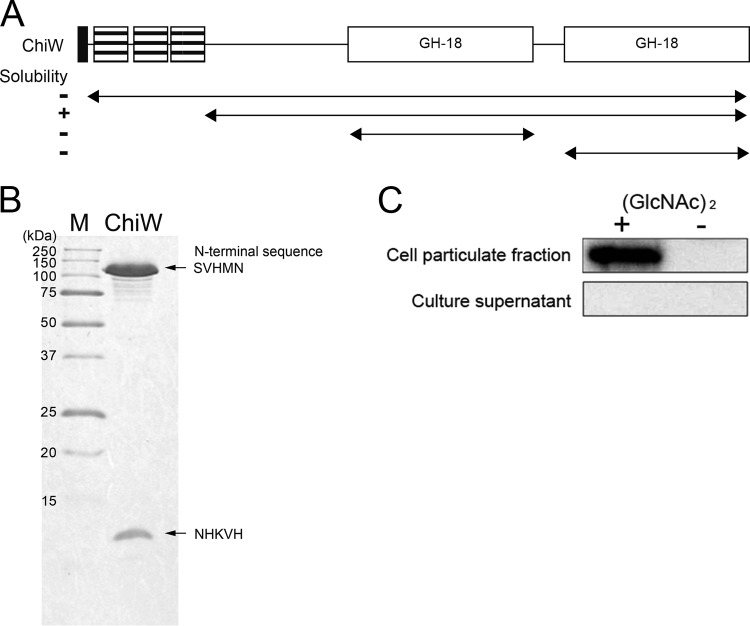
Fig 5.
Overproduction and purification of the recombinant His-tagged ChiW protein and localization of native ChiW in FPU-7 cells. (A) Arrangement of domains and deleted regions of ChiW. The solubility of the truncated mutants is indicated on the left. The ChiW with the SLH domain deleted was the only soluble form obtained. (B) SDS-PAGE of purified SLH domain deletion-containing ChiW (right lane). The purified enzyme was subjected to SDS-PAGE (15% gel). Protein bands were stained with CBB R-250. Lane M, molecular weight standards. The N-terminal sequences of the bands were SVHMN and NHKVH, as determined by protein sequencing. The site-specific proteolysis occurred between Asn282 and Ser283 during the purification step. (C) Expression and localization of ChiW in FPU-7. FPU-7 was grown in medium containing 1.0% (wt/vol) polypeptone N and 0.5% NaCl with or without 24 mM (GlcNAc)2 at pH 7.5. ChiW protein was induced by (GlcNAc)2 and was detected in the cell particulate fraction by immunoblotting.