Abstract
IgG avidity tests are used to discriminate acute from chronic infections. There are few reports on the IgG avidity profile of patients with visceral leishmaniasis (VL). This study investigated the anti-Leishmania IgG avidity in patients with classic VL (n = 10), patients showing clinical cure after treatment (n = 18), and asymptomatic subjects with at least one positive Leishmania test (n = 20). All subjects were from areas in Brazil where VL is endemic. Serum samples were collected from each subject on two different occasions. IgG avidity was evaluated by Western blotting. The proportion of high-avidity antibodies was higher in all samples from patients with classic VL. In contrast, low-avidity antibodies predominated in subjects with a history of VL, including 13 cases (72.2%) in the first assessment and 14 (77.8%) in the second. Fifteen (75%) of the asymptomatic subjects presented a predominance of low-avidity antibodies in the first assessment, and the frequency of high-avidity antibodies increased over time in seven subjects (35%) of this group. Antibodies against the 14- and/or 16-kDa antigen fraction were detected in the first assessment in all patients with classic VL, in 10 (55.5%) treated patients, and in 10 (50%) asymptomatic subjects. These were high-avidity antibodies in most cases. In the asymptomatic group, an increase in IgG avidity against the 14- and/or 16-kDa antigen fraction was observed in three cases (15%). The results indicate distinct responses in infected and asymptomatic subjects, probably associated with the length of time after infection. In this respect, IgG avidity tests represent a new approach to better characterize asymptomatic VL.
INTRODUCTION
The incidence of visceral leishmaniasis (VL) has increased each year in Brazil, despite the application of control measures advocated by the Ministry of Health. An annual average of 3,379 cases was registered between 1999 and 2008, and the mean annual incidence was 1.9 cases per 100,000 inhabitants (1). Visceral leishmaniasis exhibits a variety of clinical presentations, ranging from asymptomatic forms to classic disease characterized by fever, paleness, and splenomegaly. Asymptomatic and oligosymptomatic infections are more frequent. According to the World Health Organization, only 10 to 20% of infected subjects develop severe forms of the disease (2).
The diagnosis of asymptomatic infection with Leishmania (Leishmania) infantum has grown in importance over recent years. The true epidemiological role of asymptomatic carriers in the transmission chain of the disease is not well established, and expansion of VL might be associated with routes of infection other than vector transmission, such as transfusion-associated and congenital transmission. Furthermore, the identification of asymptomatic infection is useful for the management of patients with immunosuppressive conditions, such as patients with HIV/AIDS and patients undergoing immunomodulatory therapy, and for evaluation of the effectiveness of disease control measures.
Although different methods with good sensitivity and specificity are available for the detection of an anti-Leishmania response, the precise diagnosis of asymptomatic infection continues to be a challenge. In the first studies, the prevalence of inapparent infections was estimated based on the results of the Montenegro skin test (MST) and serological methods such as the direct agglutination test, indirect immunofluorescence test (IIFT), and enzyme-linked immunosorbent assay (ELISA) using promastigote-derived antigens (3–8). Over the last few decades, several studies have evaluated the use of recombinant antigens for the diagnosis of subclinical cases, but the results are controversial. Some investigators suggested a good sensitivity of ELISA using the recombinant K39 antigen (ELISA-rK39) in the diagnosis of cases of active VL but not cases of inapparent infection (9, 10). Other studies found asymptomatic subjects with positive ELISA-rK39 results, but follow-up did not reveal progression of these cases to classic VL (11–13).
PCR has shown higher efficacy than that of serological tests in the identification of asymptomatic cases of VL (12). However, this technique also detected positive results for subjects living in areas of endemicity but who did not progress to classic disease. In addition, there was frequent disagreement between the results of serological tests and PCR (12–16).
Studies in the literature published so far show a highly variable prevalence (0.6 to 71.3%) of asymptomatic carriers, depending on the population studied and the technique used (17). Furthermore, studies simultaneously using different techniques in the same population have reported low levels of agreement between results (12–14).
In an attempt to develop an alternative method that better characterizes asymptomatic infection, this study investigated the performance of anti-Leishmania IgG antibody avidity tests in subjects with different presentations of VL. IgG antibody avidity tests have been used to distinguish recent primary from chronic infections for diseases such as toxoplasmosis and cytomegalovirus infection (18, 19). However, there are few studies investigating IgG avidity in leishmaniasis. Unlike the reasoning we use for acute and subacute diseases, the period of incubation in leishmaniasis is usually long, leading to an increase in IgG antibody avidity during the course of the disease (20). The performance of IgG antibody avidity testing in Leishmania infection, particularly for asymptomatic cases, is not well established.
MATERIALS AND METHODS
Subjects.
For this study, serum samples collected from subjects living in the towns of Porteirinha and Janaúba, Minas Gerais, Brazil, were used. These towns are located in the northern mesoregion of Minas Gerais and are considered areas of endemicity for VL. Ninety-six serum samples (two samples per subject) were collected. The subjects were selected by convenience sampling and were divided into groups as follows.
(i) Group 1 (n = 10).
Group 1 included patients with clinical symptoms of VL whose diagnosis was confirmed by parasitological examination or serological testing combined with a specific therapeutic response (to N-methylglucamine antimoniate). These patients were evaluated in a study conducted in the town of Janaúba, and serum samples were collected before and after 6 months of specific treatment.
(ii) Group 2 (n = 18).
Group 2 included subjects with a history of VL (confirmed using the same criteria as those described above) who presented with clinical cure after specific treatment for more than 6 months. These subjects were from the town of Porteirinha. The interval between treatment and collection of the first serum sample ranged from 7 months to 12 years. Two samples were collected from each subject, and the mean interval between the first and second collections was 3 years.
(iii) Group 3 (n = 20).
Group 3 included asymptomatic subjects with at least one Leishmania-positive immunological test (MST, immunochromatographic test using the rK39 antigen, IIFT, ELISA using promastigote antigen, ELISA-rK39, or ELISA-rK26). These subjects were also from the area of Porteirinha, where VL is endemic. Two samples were also collected from each subject, and the mean interval between the first and second collections was 3 years.
Serum samples collected from subjects living in an area where VL is not endemic were tested as negative controls in all reactions. For this purpose, 10 samples were collected from blood donors seen at the Uberaba Regional Blood Center.
The biological samples were obtained according to the guidelines of Resolution 196/96 of the National Health Council, which regulates research involving humans. The serum samples tested in this study were stored at −20°C in the Laboratory of Immunology, Universidade Federal do Triângulo Mineiro (UFTM). The project was approved by the Ethics Committee of UFTM.
Anti-Leishmania IgG antibody avidity was evaluated by Western blotting using crude L. (L.) infantum antigen.
Antigen preparation.
For antigen preparation, L. (L.) infantum strain MCER/BR/79/M6445 was cultured in Schneider's medium (Sigma Chemical Co., St. Louis, MO) supplemented with 20% fetal bovine serum and 40 μg/ml gentamicin. Promastigotes grown for 2 or 3 days were washed four times in phosphate-buffered saline (PBS) by successive centrifugation at 3,200 × g for 10 min at 4°C. Parasites were then counted in a Neubauer chamber, and the concentration was adjusted to 2 × 108 promastigotes/ml.
The antigen was prepared as described by Mary et al. (21), with some modifications. The parasites were resuspended in 500 μl electrophoresis sample buffer (625 mM Tris-HCl, pH 6.8, 25% glycerol, 2% SDS, 5% 2-mercaptoethanol) and boiled for 5 min at 100°C. Next, the mixture was centrifuged at 15,000 × g for 10 min at 4°C. The supernatant was divided into aliquots and stored at −20°C until the time of use. Protein concentration was determined by the method of Bradford (22).
SDS-PAGE and Western blotting.
The antigen extract was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (23), using a 15% resolving gel and a 4% stacking gel, and 300 μg of the antigen was applied to the gel. The resolving gels measured approximately 8.5 by 6.0 cm. The gel was run at 60 V for 30 min. After the sample had penetrated the resolving gel, the voltage was maintained at 120 V until the indicator dye had reached the lower end of the gel. The Precision Plus Protein Kaleidoscope standard (Bio-Rad Laboratories, Hercules, CA) was used for comparison of molecular weights.
The proteins separated by electrophoresis were transferred to 0.45-μm nitrocellulose membranes (Bio-Rad Laboratories) in a semidry system (Trans-Blot; Bio-Rad) at 10 V for 30 min, using Towbin transfer buffer (24). After transfer, the membranes were blocked for 15 min in a solution of 5% skim milk in PBS-0.05% Tween 20 (PBS-T20) and washed three times in PBS-T20 for 5 min. Next, 4-mm strips were cut and incubated in duplicate with sera diluted 1:100 in PBS-T20 plus 0.5% skim milk for 2 h. After incubation, the strips were rapidly washed in PBS-T20, and one strip was incubated with PBS-T20 and the other with 8 M urea for 15 min. The two strips were then washed three times in PBS-T20 for 5 min and incubated for 1 h with anti-human IgG–peroxidase (DakoCytomation, Glostrup, Denmark) diluted 1:2,000 in PBS-T20 plus 0.5% skim milk. A new wash cycle was performed, and the reaction was developed with a solution of hydrogen peroxide and 3,3′-diaminobenzidine for 30 s (Sigma Chemical Co., St. Louis, MO).
The reactivity profiles of IgG antibodies to the different antigen fractions on strips treated (U+) or not treated (U−) with urea were analyzed using Image J v1.45s software (National Institutes of Health, Bethesda, MD). Each pair of strips was marked on digitized gray-scale images, and a graph with the intensity peaks corresponding to each band was constructed. IgG antibody avidity was estimated by comparing the peaks recorded for U+ and U− strips. Bands corresponding to antibodies that were no longer visible on U+ strips were classified as low-avidity bands, and those that were still present, even at a lower intensity, were classified as high-avidity bands (Fig. 1).
Fig 1.
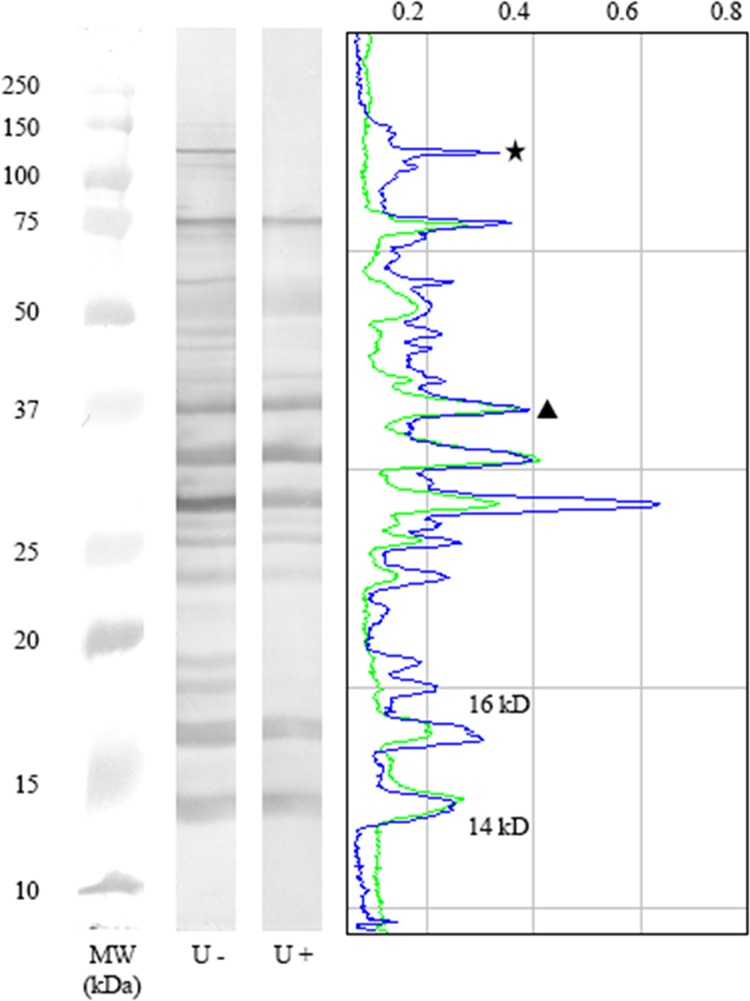
Analysis of IgG antibody avidity against crude Leishmania infantum antigen by Western blotting. Intensity peaks corresponding to each band were constructed using ImageJ software. IgG avidity was determined by comparing the reactivities on strips treated (U+) or not treated (U−) with urea. The symbols represent examples of bands with antibodies reacting with low avidity (star) and high avidity (triangle). The peaks of the 14- and 16-kDa antigen fractions are indicated in the graph. Representative strips for a seropositive asymptomatic subject (group 3) are shown. MW, molecular size in kilodaltons.
General analysis considering any degree of reactivity on the strips was performed to compare the total IgG antibody avidity profiles between the different groups. The numbers of high- and low-avidity bands were divided by the total number of bands per sample, and the results are expressed as percentages.
Statistical analysis.
The results were analyzed with GraphPad Prism v5.0 software (GraphPad Software, Inc., San Diego, CA). The percentage of bands for each avidity level was compared within each group by the Mann-Whitney test and between groups by the Kruskal-Wallis test followed by Dunn's posttest. P values of <0.05 were considered to indicate statistical significance.
RESULTS
The serum samples from patients with classic VL exhibited IgG antibody reactivity against a large number of antigen fractions with molecular masses ranging from 10 to 112 kDa. This band pattern was similar for all samples studied in this group. However, the presence of bands very close to each other impaired the determination of the molecular sizes of high-molecular-weight antigen fractions. It was possible to identify reactivity against the antigen fractions of approximately 14 and 16 kDa in serum samples from all patients with classic VL. These fractions were similar to the bands described by Mary et al. (in 1992), which showed 100% sensitivity and 98% specificity in the diagnosis of VL in that study (21). We therefore performed two analyses. In the first analysis, any degree of reactivity on the strips was considered, and IgG antibody avidity was evaluated by comparing the relative numbers of low- and high-avidity bands for each serum sample tested. In the second analysis, IgG antibody avidity against the 14- and 16-kDa antigen fractions was evaluated separately.
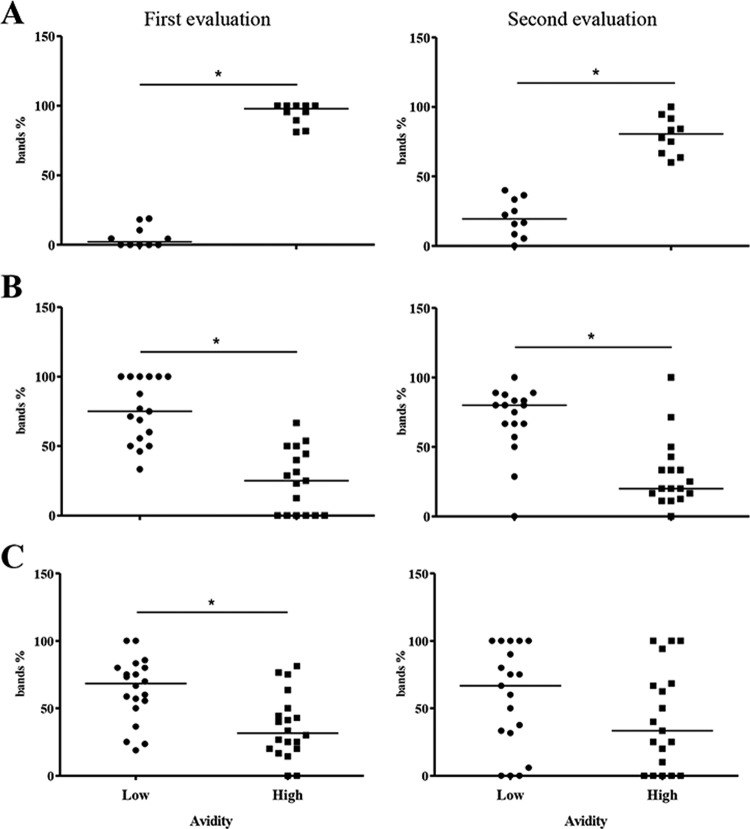
In group 1, the frequency of high-avidity antibodies was significantly higher than that of low-avidity antibodies in all cases, including serum samples collected before and after 6 months of specific treatment (P < 0.001) (Fig. 2A). Reactivity against the 14- and 16-kDa antigen fractions was observed in all samples from patients with classic VL in the two assessments. As observed in the analysis of total IgG avidity, there was a predominance of high-avidity antibodies against the two antigen fractions. Only one subject (10%) had low-avidity antibodies against the 14-kDa fraction in the two assessments and against the 16-kDa fraction in the second assessment.
Fig 2.
Evaluation of IgG antibody avidity in patients with classic VL (A), subjects with a history of VL (B), and infected asymptomatic subjects (C) according to the percentage of bands corresponding to low-avidity or high-avidity antibodies. Each symbol represents the value for one individual. The bars indicate the median values for the groups (*, P < 0.05).
Analysis of serum samples from subjects with a history of VL (group 2) showed a highly variable reactivity profile, with the observation of a smaller number of bands than that for group 1. However, only two samples were classified as nonreactive, one of which was in the first assessment and the other of which was in the second assessment. Analysis of total IgG avidity showed a predominance of low-avidity antibodies in serum samples from 13 subjects (72.2%) in the first assessment and from 14 subjects (77.8%) in the second assessment. In the two assessments, the mean frequencies of low-avidity bands were significantly higher than those of high-avidity bands (P < 0.001) (Fig. 2B).
Also in group 2, antibodies against the 14-kDa antigen fraction were detected in samples from seven subjects (38.9%) in the first assessment, all of them high-avidity antibodies. In the second assessment, only one subject (5.5%) continued to present reactivity against this antigen fraction (low-avidity antibodies). Reactivity against the 16-kDa fraction was observed in nine subjects (50%) in the first assessment. In eight of these cases, the antibodies had high avidity. In the second assessment, reactivity against this antigen fraction was observed in samples from six subjects (33.3%), including three with low-avidity antibodies and three with high-avidity antibodies.
In the group of asymptomatic subjects (group 3), larger proportions of low-avidity IgG antibodies were observed in serum samples from 15 subjects (75%) in the first assessment, with a significant difference in the relative numbers of low- and high-avidity bands (P = 0.003) (Fig. 2C). In the second assessment, low-avidity IgG antibodies continued to predominate in samples from 11 subjects (55%). However, an increase in the proportion of high-avidity bands compared to the first assessment was observed in seven subjects (35%). In these cases, the band pattern was similar to that seen for patients with classic VL, whereas for subjects presenting a predominance of low-avidity antibodies, the pattern was similar to that observed for subjects with a history of the disease.
Antibodies against the 14-kDa antigen fraction were detected in samples from eight subjects (40%) of group 3 in the first assessment, with the presence of high-avidity antibodies in seven cases. Ten samples (50%) were reactive against the 16-kDa fraction, with the observation of high-avidity antibodies in five and low-avidity antibodies in the other five. In the second assessment, serum samples from 10 subjects (50%) contained antibodies against the 14-kDa antigen fraction; these were high-avidity antibodies in most cases (n = 8). Reactivity against the 16-kDa fraction was observed in 12 cases (60%), including 8 with high-avidity antibodies and 4 with low-avidity antibodies.
Comparison of the percentages of bands corresponding to low-avidity antibodies between the three groups showed a significant difference between group 1 and the other groups in the two assessments, with the frequency of low-avidity antibodies being significantly lower in group 1 (classic VL) (P < 0.0001 and P = 0.0029) (Fig. 3).
Fig 3.
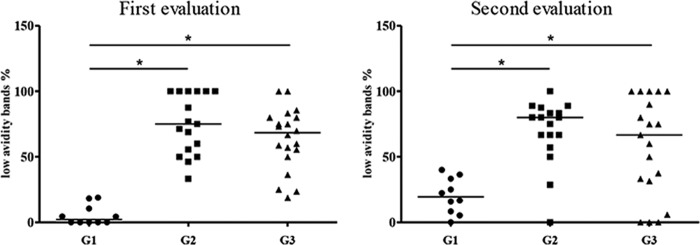
Comparison of low-avidity antibodies in patients with classic VL (G1), subjects with a history of VL (G2), and asymptomatic infected subjects (G3) in both assessments. Each symbol represents the value for one individual. The bars indicate the median values for the groups (*, P < 0.05).
DISCUSSION
Since the 1980s, Brazil has witnessed a significant increase in the number of human and canine cases of VL. Control strategies advocated by the Ministry of Health have been shown to be poorly effective because of the epidemiological characteristics of the disease and the still insufficient knowledge of the different elements that comprise its transmission chain (25). One of the challenges encountered is the identification of cases of asymptomatic VL. Within this context, the objective of this study was to characterize the performance of an anti-Leishmania IgG antibody avidity test in seropositive subjects with different presentations of the disease. We believe that the IgG avidity test would be useful for characterizing the activity of Leishmania infection, thus extending the application of this strategy, which is generally used to estimate the time of infection.
IgG avidity was evaluated by Western blotting. This technique was chosen because it permits the investigation of IgG antibody avidity against different antigen fractions. However, we found a large number of bands that were very close to each other, a fact impairing the calculation of all molecular weights and the comparison of the most frequent antigen fractions between groups. We therefore analyzed the total avidity of each sample by considering the levels of avidity of most bands. In addition, bands of 14 and 16 kDa were analyzed, as these were identified by Western blotting in all subjects with classic VL. These fractions had shown a specificity of 97.3% and a positive likelihood ratio of 20.0 in a previous study from our group which included 57 samples from patients with VL, negative controls, and patients with other diseases (unpublished data). In addition, these fractions are compatible with bands described by Mary et al. (21) and cited in several studies on the diagnosis of VL (26–29).
High-avidity IgG antibodies predominated in patients with classic VL both upon general analysis of the bands and when the 14- and 16-kDa fractions were analyzed separately. This finding is consistent with the long incubation period generally observed in the disease and agrees with the findings of Redhu et al., who detected high-avidity IgG antibodies against the recombinant r-KE-16 antigen by ELISA in an area where L. (L.) donovani is endemic. In that study, conducted in India, the authors evaluated the risk of developing post-kala-azar dermal leishmaniasis and found that the increase in IgG antibody avidity was associated with the duration of the disease (20). In contrast, in tegumentary leishmaniasis, high-avidity antibodies were detected in patients with recent lesions (duration of ≤3 months) (30).
Unexpectedly, a predominance of low-avidity antibodies was observed in patients receiving specific treatment for VL for more than 6 months, a finding that persisted over time and that was not associated with clinical symptoms of VL. In an analogous manner, for tegumentary leishmaniasis, de Souza et al. detected low-avidity antibodies in patients with a history of lesions of up to 10 years (30).
It is possible that the low-avidity antibodies observed in the present study correspond to nonspecific reactivity, since IgG antibodies against the 14- and 16-kDa antigen fractions exhibited high avidity in most cases. A reduction in reactivity against these antigen fractions was observed in the second assessment, suggesting that the time after clinical cure may be associated with a loss of reactivity to these bands. Low-avidity IgG antibodies against the 14- and/or 16-kDa fraction were detected in three treated patients in the second assessment. In these cases, it is possible that the reduction in the level of antibodies against these specific antigens resulted in a weaker binding to the membrane and, consequently, more susceptibility to disruption by urea, which would be interpreted as low avidity.
Analysis of serum samples from asymptomatic subjects revealed two subgroups with different profiles of total IgG antibody avidity. A predominance of low-avidity antibodies was observed in the first subgroup, similar to the case for subjects receiving treatment, whereas avidity increased over time in the second subgroup. The fact that none of the subjects developed classic disease during the period studied suggests the presence of an immune response pattern that is able to maintain the parasite-host balance. However, the increase in avidity over time may be indicative of the occurrence of active infection. In addition to the increase in total IgG avidity, an increase in avidity against the 14- and/or 16-kDa fraction was observed in three cases. Thus, IgG avidity against these antigens may be a potential marker to evaluate the activity of infection. To confirm this hypothesis, it would be necessary to evaluate a larger number of asymptomatic subjects over shorter intervals, which was not done in the present study.
The limitations of this study are the relatively small number of samples and the use of minigels, which can compromise the analysis of specific antigen fractions of high molecular weight.
Finally, the present results suggest that subjects with asymptomatic infection in whom conversion from low- to high-avidity IgG occurs are carriers of active infection. This group is therefore at an increased risk of developing the disease under conditions of immunosuppression. Moreover, this group is important from an epidemiological point of view as a potential reservoir of Leishmania for both the vector and other possible routes of transmission, such as transfusion-associated transmission. We conclude that IgG avidity tests are a promising approach to characterize asymptomatic VL.
ACKNOWLEDGMENTS
We thank the National Council for Scientific and Technological Development for MGST fellowships (master's degree, Programa de Pós-Graduação em Medicina Tropical e Infectologia, UFTM).
Footnotes
Published ahead of print 4 September 2013
REFERENCES
- 1.Ministério da Saúde. 2009. Guia de vigilância epidemiológica, 7th ed. Ministério da Saúde, Brasília, Brazil [Google Scholar]
- 2.Alvar J, Canavate C, Gutierrez-Solar B, Jimenez M, Laguna F, Lopez-Velez R, Molina R, Moreno J. 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin. Microbiol. Rev. 10:298–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harith AE, Kolk AH, Kager PA, Leeuwenburg J, Faber FJ, Muigai R, Kiugu S, Laarman JJ. 1987. Evaluation of a newly developed direct agglutination test (DAT) for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis: comparison with IFAT and ELISA. Trans. R. Soc. Trop. Med. Hyg. 81:603–606 [DOI] [PubMed] [Google Scholar]
- 4.Evans TG, Teixeira MJ, McAuliffe IT, Vasconcelos I, Vasconcelos AW, Sousa Ade A, Lima JW, Pearson RD. 1992. Epidemiology of visceral leishmaniasis in northeast Brazil. J. Infect. Dis. 166:1124–1132 [DOI] [PubMed] [Google Scholar]
- 5.Shiddo SA, Akuffo HO, Mohamed AA, Huldt G, Nilsson LA, Ouchterlony O, Thorstensson R. 1995. Visceral leishmaniasis in Somalia: prevalence of leishmanin-positive and seropositive inhabitants in an endemic area. Trans. R. Soc. Trop. Med. Hyg. 89:21–24 [DOI] [PubMed] [Google Scholar]
- 6.D'Oliveira Junior A, Costa SR, Barbosa AB, Orge Mdl Carvalho GEM 1997. Asymptomatic Leishmania chagasi infection in relatives and neighbors of patients with visceral leishmaniasis. Mem. Inst. Oswaldo Cruz 92:15–20 [DOI] [PubMed] [Google Scholar]
- 7.Corredor Arjona A, Alvarez Moreno CA, Agudelo CA, Bueno M, Lopez MC, Caceres E, Reyes P, Duque Beltran S, Gualdron LE, Santacruz MM. 1999. Prevalence of Trypanosoma cruzi and Leishmania chagasi infection and risk factors in a Colombian indigenous population. Rev. Inst. Med. Trop. Sao Paulo 41:229–234 [DOI] [PubMed] [Google Scholar]
- 8.Jeronimo SM, Teixeira MJ, Sousa A, Thielking P, Pearson RD, Evans TG. 2000. Natural history of Leishmania (Leishmania) chagasi infection in northeastern Brazil: long-term follow-up. Clin. Infect. Dis. 30:608–609 [DOI] [PubMed] [Google Scholar]
- 9.Badaro R, Benson D, Eulalio MC, Freire M, Cunha S, Netto EM, Pedral-Sampaio D, Madureira C, Burns JM, Houghton RL, David JR, Reed SG. 1996. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J. Infect. Dis. 173:758–761 [DOI] [PubMed] [Google Scholar]
- 10.Braz RF, Nascimento ET, Martins DR, Wilson ME, Pearson RD, Reed SG, Jeronimo SM. 2002. The sensitivity and specificity of Leishmania chagasi recombinant K39 antigen in the diagnosis of American visceral leishmaniasis and in differentiating active from subclinical infection. Am. J. Trop. Med. Hyg. 67:344–348 [DOI] [PubMed] [Google Scholar]
- 11.Nascimento MDSB, Neto APBN, Silva NL, Coaracy GAV, Souza EC, Silva MH, Lindoso E, Sousa WB, Magalhães CA, Brito GO, Almeida PEA, Bezerra GFB, Viana GMC. 2000. Progressão da intradermorreação de Montenegro e a sororreatividade por ELISA com os antígenos rk39 e crude em indivíduos assintomáticos de área endêmica de leishmaniose visceral. Rev. Soc. Bras. Med. Trop. 33(Suppl 1):41 [Google Scholar]
- 12.Moreno EC, Goncalves AV, Chaves AV, Melo MN, Lambertucci JR, Andrade AS, Negrao-Correa D, de Figueiredo Antunes CM, Carneiro M. 2009. Inaccuracy of enzyme-linked immunosorbent assay using soluble and recombinant antigens to detect asymptomatic infection by Leishmania infantum. PLoS Negl. Trop. Dis. 3:e536. 10.1371/journal.pntd.0000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva LA, Romero HD, Nogueira Nascentes GA, Costa RT, Rodrigues V, Prata A. 2011. Antileishmania immunological tests for asymptomatic subjects living in a visceral leishmaniasis-endemic area in Brazil. Am. J. Trop. Med. Hyg. 84:261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero HD, Silva Lde A, Silva-Vergara ML, Rodrigues V, Costa RT, Guimaraes SF, Alecrim W, Moraes-Souza H, Prata A. 2009. Comparative study of serologic tests for the diagnosis of asymptomatic visceral leishmaniasis in an endemic area. Am. J. Trop. Med. Hyg. 81:27–33 [PubMed] [Google Scholar]
- 15.Silva LA, Romero HD, Fagundes A, Nehme N, Fernandes O, Rodrigues V, Costa RT, Prata A. 2013. Use of the polymerase chain reaction for the diagnosis of asymptomatic Leishmania infection in a visceral leishmaniasis-endemic area. Rev. Inst. Med. Trop. Sao Paulo 55:101–104 [DOI] [PubMed] [Google Scholar]
- 16.Moreno EC, Melo MN, Lambertucci JR, Serufo JC, Andrade AS, Antunes CM, Genaro O, Carneiro M. 2006. Diagnosing human asymptomatic visceral leishmaniasis in an urban area of the State of Minas Gerais, using serological and molecular biology techniques. Rev. Soc. Bras. Med. Trop. 39:421–427 [DOI] [PubMed] [Google Scholar]
- 17.Michel G, Pomares C, Ferrua B, Marty P. 2011. Importance of worldwide asymptomatic carriers of Leishmania infantum (L. chagasi) in human. Acta Trop. 119:69–75 [DOI] [PubMed] [Google Scholar]
- 18.Camargo ME, da Silva SM, Leser PG, Granato CH. 1991. Avidity of specific IgG antibodies as markers of recent primary infection caused by Toxoplasma gondii. Rev. Inst. Med. Trop. Sao Paulo 33:213–218 [PubMed] [Google Scholar]
- 19.Lazzarotto T, Spezzacatena P, Pradelli P, Abate DA, Gabrielli L, Varani S, Landini MP. 1998. Cytomegalovirus infection in pregnancy: a still complicated diagnostic problem. Intervirology 41:149–157 [DOI] [PubMed] [Google Scholar]
- 20.Redhu NS, Dey A, Balooni V, Singh S. 2006. Use of immunoglobulin G avidity to determine the course of disease in visceral and post-kala-azar dermal leishmaniasis patients. Clin. Vaccine Immunol. 13:969–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mary C, Lamouroux D, Dunan S, Quilici M. 1992. Western blot analysis of antibodies to Leishmania infantum antigens: potential of the 14-kD and 16-kD antigens for diagnosis and epidemiologic purposes. Am. J. Trop. Med. Hyg. 47:764–771 [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 23.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 24.Towbin H, Staehelin T, Gordon J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministério da Saúde. 2006. Manual de vigilância e controle da leishmaniose visceral. Ministério da Saúde, Brasília, Brazil [Google Scholar]
- 26.Marty P, Lelievre A, Quaranta JF, Rahal A, Gari-Toussaint M, Le Fichoux Y. 1994. Use of the leishmanin skin test and Western blot analysis for epidemiological studies in visceral leishmaniasis areas: experience in a highly endemic focus in Alpes-Maritimes (France). Trans. R. Soc. Trop. Med. Hyg. 88:658–659 [DOI] [PubMed] [Google Scholar]
- 27.Le Fichoux Y, Quaranta JF, Aufeuvre JP, Lelievre A, Marty P, Suffia I, Rousseau D, Kubar J. 1999. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in southern France. J. Clin. Microbiol. 37:1953–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mary C, Faraut F, Drogoul MP, Xeridat B, Schleinitz N, Cuisenier B, Dumon H. 2006. Reference values for Leishmania infantum parasitemia in different clinical presentations: quantitative polymerase chain reaction for therapeutic monitoring and patient follow-up. Am. J. Trop. Med. Hyg. 75:858–863 [PubMed] [Google Scholar]
- 29.Riera C, Fisa R, Lopez-Chejade P, Serra T, Girona E, Jimenez M, Muncunill J, Sedeno M, Mascaro M, Udina M, Gallego M, Carrio J, Forteza A, Portus M. 2008. Asymptomatic infection by Leishmania infantum in blood donors from the Balearic Islands (Spain). Transfusion 48:1383–1389 [DOI] [PubMed] [Google Scholar]
- 30.de Souza MA, da Silva AG, Afonso-Cardoso SR, Favoreto SJ, Ferreira MS. 2005. Immunoglobulin isotype and IgG subclass profiles in American tegumentary leishmaniasis. Rev. Soc. Bras. Med. Trop. 38:137–141 [DOI] [PubMed] [Google Scholar]