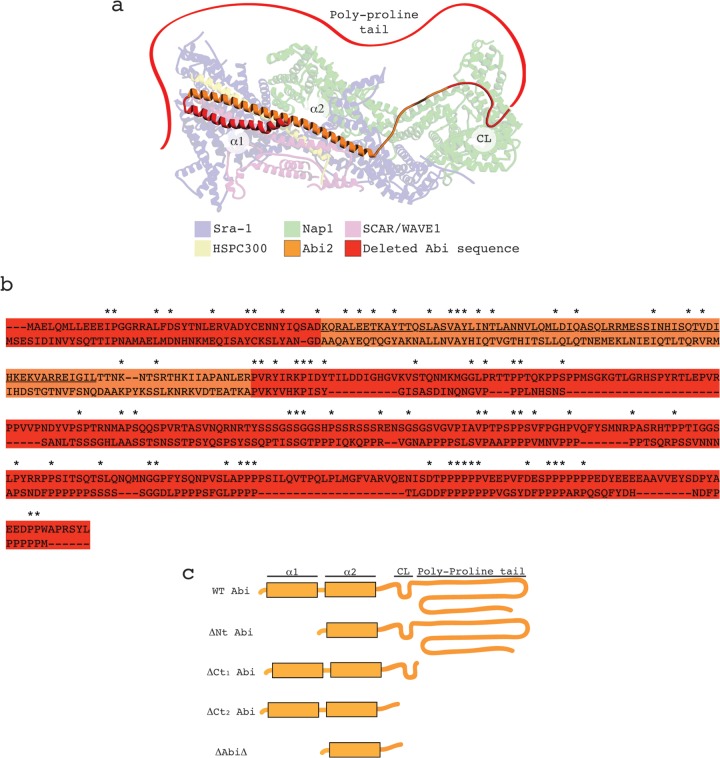
Fig 1.
Design and implementation of Abi deletion series. (a) Illustration of human SCAR/WAVE1 complex derived from the recently resolved crystal structure available from the Protein Data Bank (accession number 3P8C). The sequence corresponding to that deleted in the Dictyostelium Abi is highlighted in red. Red ribbon, the Abi2 polyproline region that is absent from the crystal structure (not to scale). Labels designate different domains/features of Abi2: α1, 1st alpha helix; α2, 2nd alpha helix; CL, conserved loop. (b) Protein alignment between human Abi2 (above), represented in panel a, and Dictyostelium Abi (below) with a color scheme identical to that described for panel a. The sequence corresponding to the C-terminal SH3 domain of human Abi2 was removed to aid alignment, as Dictyostelium Abi does not possess this domain. Asterisks, amino acid identities; underlined sequence, amino acids corresponding to the 2nd alpha helix in panel a. (c) Diagrammatic representation of the Dictyostelium Abi domain structure and the subsequent deletion series undertaken. WT, full-length Abi; ΔNt, N-terminally truncated Abi; ΔCt1 and ΔCt2, C-terminally truncated Abi as shown; ΔAbiΔ, combination of both N-terminally and C-terminally truncated Abi.