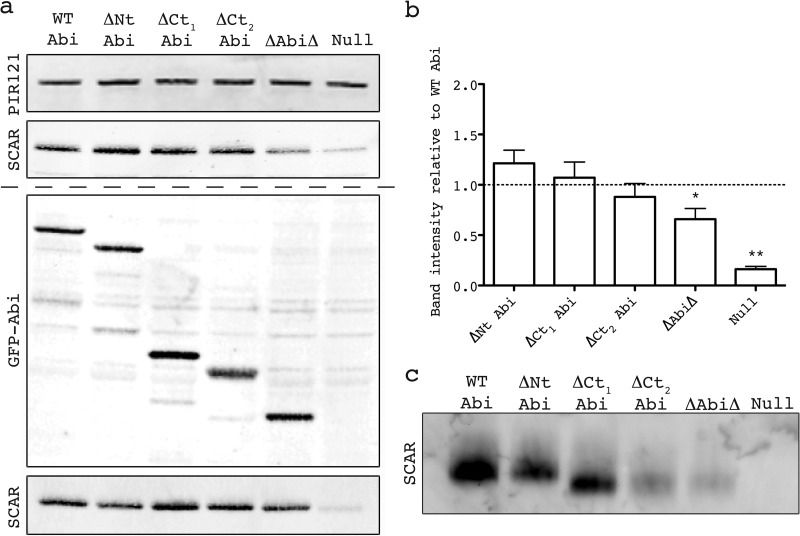
Fig 2.
Identification of minimal Abi fragment. (a) Western blot revealing stabilization of SCAR by the untagged/tagged Abi deletion series transformed into an abiA-null strain. (Top) Lysates probed with antibody against PIR121, a stable member of the complex that was used as a loading control; (second panel) lysates probed with anti-SCAR antibody demonstrating stabilization of SCAR by different untagged Abi fragments; (third panel) expression of truncated GFP-tagged Abi constructs confirmed by anti-GFP Western blotting; (bottom) lysates probed with anti-SCAR antibody demonstrating stabilization of SCAR by different GFP-tagged Abi fragments. The dashed line divides blots into those derived from cells that possessed untagged Abi deletion series (above the dashed line) and those that possessed GFP-tagged Abi deletion series (below the dashed line). (b) Quantification of SCAR levels was achieved by normalization of the SCAR band intensity to the PIR121 band intensity for each untagged transformant over four blots. *, significantly different from WT Abi SCAR levels (unpaired t test, P < 0.05); **, significantly different from ΔAbiΔ SCAR levels (unpaired t test, P < 0.0001). Error bars indicate SEMs. (c) Native PAGE demonstrating the stabilization of the entire SCAR complex by the untagged Abi fragments. Intact protein complexes were separated by native PAGE and probed with anti-SCAR antibody following Western blotting. WT, full-length wild-type Abi; ΔNt, N-terminally truncated Abi; ΔCt1 and ΔCt2, C-terminally truncated Abi as shown in Fig. 1c; ΔAbiΔ, combination of both N-terminally and C-terminally truncated Abi; Null, HSPC300-GFP-only vector/empty GFP vector.