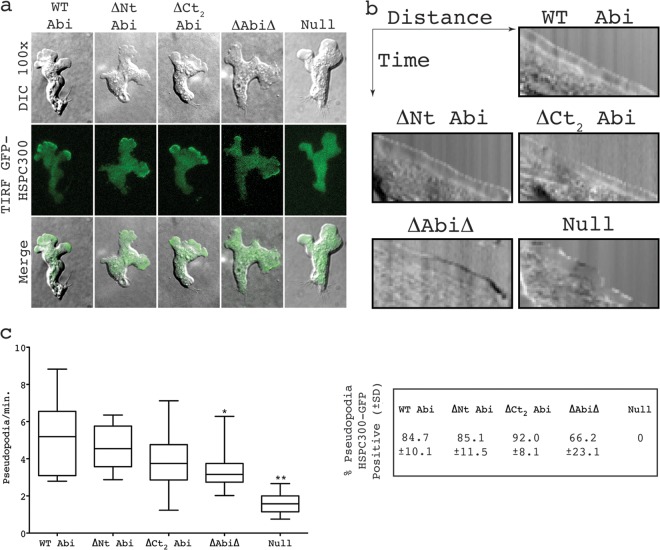
Fig 4.
SCAR complexes containing truncated Abi proteins localize normally in migrating cells. (a) Localization of HSPC300-GFP in abiA-null cells expressing different Abi fragments. (Top row) DIC images of migrating cells coexpressing Abi-truncated constructs and the SCAR complex marker HSPC300-GFP; (middle row) TIRF images of the same cells revealing localization of mutant SCAR complexes; (bottom row) merged images of the upper two rows. (b) Representative kymographs highlighting smooth progression of advancing protrusions in cells expressing Abi truncations. Null cells present with a ragged slope indicative of blebbing. (c) All truncated Abi proteins rescue the suppressed pseudopod formation of abiA-null cells. *, significantly reduced rate of pseudopod formation compared to that for WT Abi-rescued cells (unpaired t test, P < 0.05); **, significantly reduced rate of pseudopod formation compared to that for ΔAbiΔ-rescued cells (unpaired t test, P < 0.001). The percentages in the box on the right indicate the proportion of pseudopodia with robust HSPC300-GFP localization ± SD. WT Abi, full-length Abi; ΔNt, N-terminally truncated Abi; ΔCt2, C-terminally truncated Abi; ΔAbiΔ, combination of both N-terminally and C-terminally truncated Abi; Null, HSPC300-GFP-only vector.