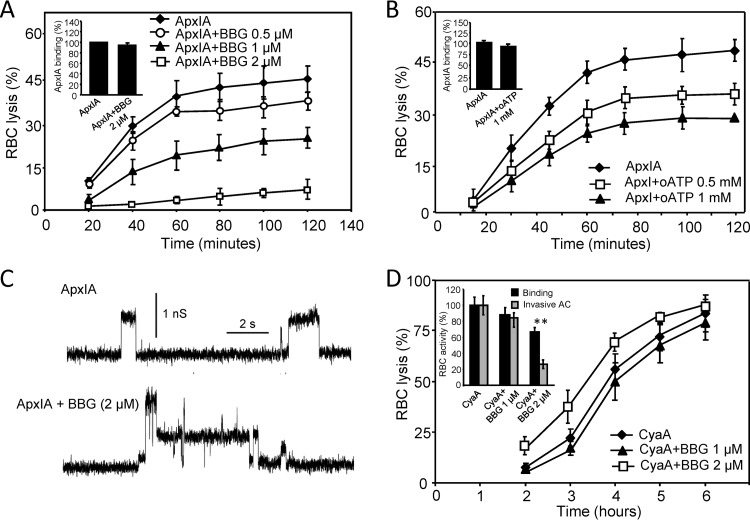
Fig 4.
ApxIA-induced hemolysis is inhibited by selective P2X7 antagonist BBG or oATP. Sheep erythrocytes (5 × 108/ml) in HBSS buffer were preincubated in the presence or absence of BBG (A) or oATP (B) for 5 or 60 min, respectively. Hemolytic activity was measured at 37°C in the presence of 12.5 nM ApxIA as the amount of released hemoglobin by photometric determination. Sheep erythrocytes (5 × 107/ml) in HBSS buffer in the presence of 75 mM sucrose were incubated with 12.5 nM ApxIA labeled with Dyomics 647 dye in the presence or absence of purinergic antagonists at 37°C. (A and B, insets) After 120 min, cells were washed repeatedly to remove unbound ApxIA and used to determine the amount of cell-associated toxin by FACS. The binding activity of ApxIA-Dyomics 647 dye in the absence of purinergic antagonist was taken to be 100%. (C) Typical current traces of ApxIA in planar lipid bilayers in the presence or absence of BBG (2 μM) in 1 M KCl, 10 mM Tris, and 2 mM CaCl2 (pH 7.4). The toxin concentration was 2.3 nM. The applied membrane potential was 55 mV; the temperature was 25°C. (D) Sheep erythrocytes (5 × 108/ml) in HBSS buffer were preincubated for 5 min in the presence or absence of BBG. Hemolytic activity was measured in the presence of 50 nM CyaA as described for panels A and B. (Inset) Cell-binding and cell-invasive activities of CyaA in the presence of BBG. Sheep erythrocytes (5 × 108/ml) in HBSS buffer were incubated with 50 nM CyaA at 37°C in the presence or absence of BBG. After 30 min, aliquots of cell suspensions were washed repeatedly to remove unbound CyaA and used to determine the amount of cell-associated and cell-invasive AC enzyme activities. The activity of CyaA in the absence of BBG was taken to be 100%. The results represent average values from at least four independent experiments. **, statistically significant differences (P < 0.001) from the activities of CyaA without inhibitor.