Abstract
Neuroblastoma-glioma hybrid cells of line 108CC-5 were found to contain high levels of soluble adenosine 3',5'-cyclic monophosphate (cAMP)-dependent protein kinase activity and high levels of two specific cAMP receptor proteins, RI and RII. Treatment of the hybrid cells with dibutyryl cAMP increased the level of RI but did not significantly affect the level either of RII or of cAMP-dependent protein kinase activity. The effect of dibutyryl cAMP could be mimicked by prostaglandin E1 and 3-isobutyl-1-methylxanthine, both of which are known to raise cAMP levels in neuroblastoma-glioma hybrid cells. Both in control as well as in dibutyryl cAMP-treated cells, RII but not RI was associated with cAMP-dependent protein kinase. Several lines of evidence suggest that RI represents the free regulatory subunit of type I cAMP-dependent protein kinase. The presence of this regulatory subunit as free cAMP receptor protein in neuroblastoma-glioma hybrid cells may be of significance with respect to the regulation of growth and differentiation in tumor cells.
Full text
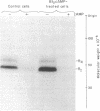
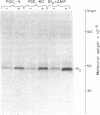
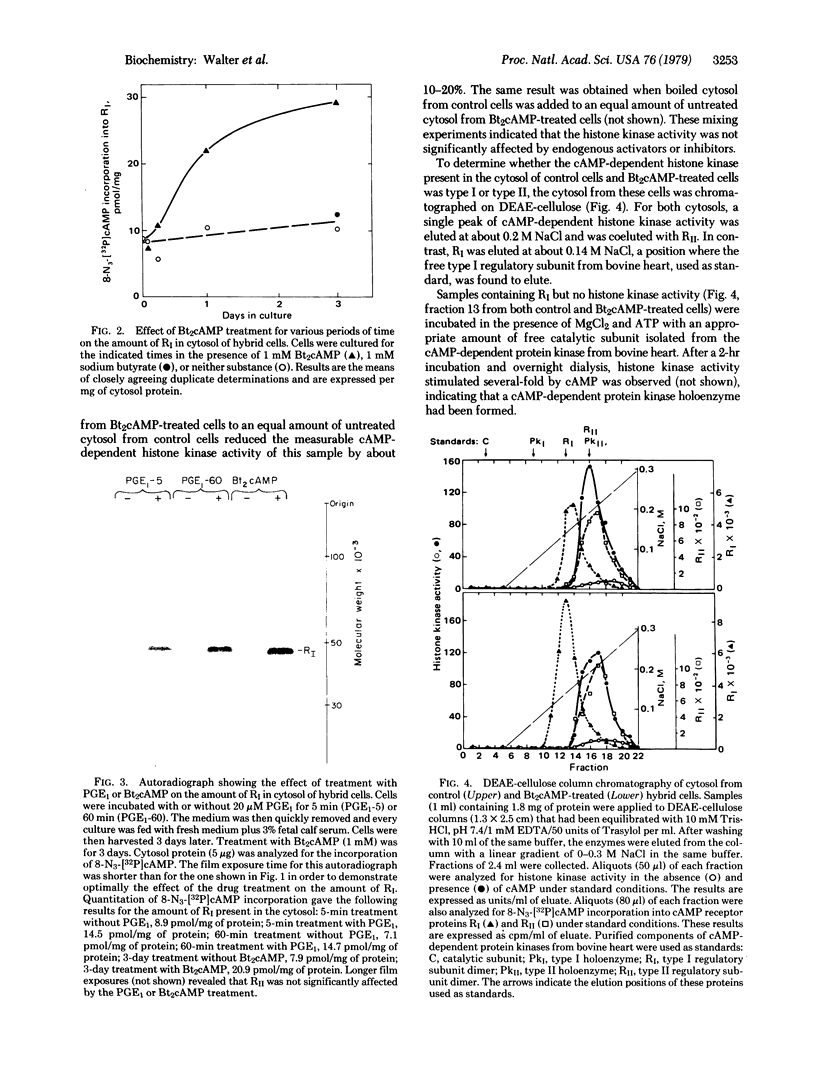
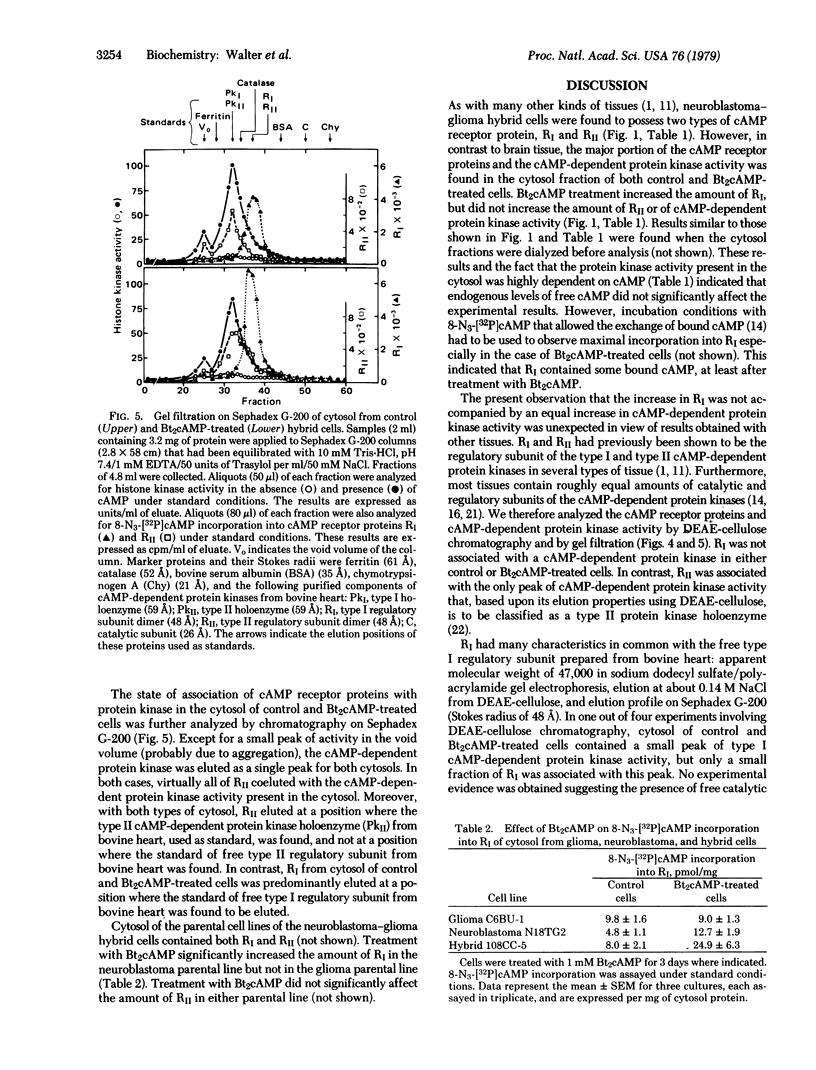
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Hamprecht B., Kemper W. High activity of choline acetyltransferase induced in neuroblastoma x glia hybrid cells. Exp Cell Res. 1974 Apr;85(2):399–408. doi: 10.1016/0014-4827(74)90142-6. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Bodwin J. S., Clair T., Cho-Chung Y. S. Inverse relation between estrogen receptors and cyclic adenosine 3':5'-monophosphate-binding proteins in hormone-dependent mammary tumor regression due to dibutyryl cyclic adenosine 3':5'-monophosphate treatment or ovariectomy. Cancer Res. 1978 Oct;38(10):3410–3413. [PubMed] [Google Scholar]
- Christian C. N., Nelson P. G., Peacock J., Nirenberg M. Synapse formation between two clonal cell lines. Science. 1977 May 27;196(4293):995–998. doi: 10.1126/science.193191. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., Lincoln T. M., Keely S. L. Compartmentalization of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in heart tissue. J Biol Chem. 1977 Jun 10;252(11):3854–3861. [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., West L., Flockhart D. A., Lincoln T. M., McCarthy D. Studies on the properties and mode of action of the purified regulatory subunit of bovine heart adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1978 Jun 10;253(11):3997–4003. [PubMed] [Google Scholar]
- Hamprecht B. Neuron models. Angew Chem Int Ed Engl. 1976 Apr;15(4):194–206. doi: 10.1002/anie.197601941. [DOI] [PubMed] [Google Scholar]
- Hamprecht B., Schultz J. Influence of noradrenalin, prostaglandin E1 and inhibitors of phosphodiesterase activity on levels of the cyclic adenosine 3':5'-monophosphate in somatic cell hybrids. Hoppe Seylers Z Physiol Chem. 1973 Dec;354(12):1633–1641. doi: 10.1515/bchm2.1973.354.2.1633. [DOI] [PubMed] [Google Scholar]
- Hamprecht B., Traber J. Dopamine-beta-hydroxylase activity in cholinergic neuroblastoma times glioma hybrid cells; increase of activity by N6,O2'-dibutyryl adenosine 3':5'-cyclic monophosphate. FEBS Lett. 1974 Jun 1;42(2):221–226. doi: 10.1016/0014-5793(74)80790-8. [DOI] [PubMed] [Google Scholar]
- Heumann R., Valet G., Maison D., Kemper J., Reiser G., Hamprecht B. Influence of the time in culture on cellular and neuronal properties of neuroblastoma x glioma hybrid cells. With an appendix, mathematical description of the kinetics of the loss in cell volume. J Cell Sci. 1977;27:141–155. doi: 10.1242/jcs.27.1.141. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Jungmann R. A., Russell D. H. Cyclic AMP, cyclic AMP-dependent protein kinase, and the regulation of gene expression. Life Sci. 1977 Jun 1;20(11):1787–1797. doi: 10.1016/0024-3205(77)90213-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lohmann S. M., Walter U., Greengard P. Protein kinases in developing rat brain. J Cyclic Nucleotide Res. 1978 Dec;4(6):445–452. [PubMed] [Google Scholar]
- McGee R., Simpson P., Christian C., Mata M., Nelson P., Nirenberg M. Regulation of acetylcholine release from neuroblastoma x glioma hybrid cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1314–1318. doi: 10.1073/pnas.75.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minna J., Glazer D., Nirenberg M. Genetic dissection of neural properties using somatic cell hybrids. Nat New Biol. 1972 Feb 23;235(60):225–231. doi: 10.1038/newbio235225a0. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Christian C. N., Daniels M. P., Henkart M., Bullock P., Mullinax D., Nirenberg M. Formation of synapses between cells of a neuroblastoma X glioma hybrid clone and mouse myotubes. Brain Res. 1978 May 26;147(2):245–259. doi: 10.1016/0006-8993(78)90838-7. [DOI] [PubMed] [Google Scholar]
- Nelson P., Christian C., Nirenberg M. Synapse formation between clonal neuroblastoma X glioma hybrid cells and striated muscle cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):123–127. doi: 10.1073/pnas.73.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Hormonal control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1977;8:145–266. [PubMed] [Google Scholar]
- Prasad K. N., Sahu S. K., Sinha P. K. Cyclic nucleotides in the regulation of expression of differentiated functions in neuroblastoma cells. J Natl Cancer Inst. 1976 Sep;57(3):619–631. doi: 10.1093/jnci/57.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. N., Sinha P. K., Sahu S. K., Brown J. L. Binding of cyclic nucleotides with soluble proteins increases in "differentiated" neuroblastoma cells in culture. Biochem Biophys Res Commun. 1975 Sep 2;66(1):131–138. doi: 10.1016/s0006-291x(75)80304-4. [DOI] [PubMed] [Google Scholar]
- Prashad N., Wischmeyer B., Evetts C., Baskin F., Rosenberg R. Dibutyryl cAMP-induced protein changes in differentiating mouse neuroblastoma cells. Cell Differ. 1977 Aug;6(2):147–157. doi: 10.1016/0045-6039(77)90036-7. [DOI] [PubMed] [Google Scholar]
- Walter U., Greengard P. Quantitative labeling of the regulatory subunit of type II cAMP-dependent protein kinase from bovine heart by a photoaffinity analog. J Cyclic Nucleotide Res. 1978 Dec;4(6):437–444. [PubMed] [Google Scholar]
- Walter U., Kanof P., Schulman H., Greengard P. Adenosine 3':5'-monophosphate receptor proteins in mammalian brain. J Biol Chem. 1978 Sep 10;253(17):6275–6280. [PubMed] [Google Scholar]
- Walter U., Uno I., Liu A. Y., Greengard P. Identification, characterization, and quantitative measurement of cyclic AMP receptor proteins in cytosol of various tissues using a photoaffinity ligand. J Biol Chem. 1977 Sep 25;252(18):6494–6500. [PubMed] [Google Scholar]