Abstract
Highly divergent vaccine-derived polioviruses (VDPVs) have been isolated from sewage in Tallinn, Estonia, since 2002. Sequence analysis of VDPVs of serotypes 2 and 3 showed that they shared common noncapsid region recombination sites, indicating origination from a single trivalent oral polio vaccine dose, estimated to have been given between 1986 and 1998. The sewage isolates closely resemble VDPVs chronically excreted by persons with common variable immunodeficiency, but no chronic excretors have yet been identified in Estonia.
TEXT
Estonia was one of the first countries in the world to eliminate endemic polio, with the last case reported in 1961 (1). The core strategies for poliomyelitis control in Estonia and worldwide include polio vaccination at high rates of coverage, sensitive surveillance for cases of acute flaccid paralysis (AFP), and virologic characterization of poliovirus isolates (2). AFP surveillance is supplemented in 20 European countries and elsewhere by environmental surveillance (3–5).
Estonia switched from oral poliovirus vaccine (OPV) to inactivated poliovirus vaccine (IPV) in 2008 (http://www.who.int/immunization_monitoring/data/est.pdf). Key factors leading to the switch were the rare clinical consequences of the genetic instability of OPV (6): the occurrence of vaccine-associated paralytic poliomyelitis (VAPP) in OPV recipients or contacts (7) and the emergence of genetically divergent vaccine-derived polioviruses (VDPVs) (8). VDPVs are defined as having >1% divergence (types 1 and 3) or >0.6% divergence (type 2) from the corresponding OPV strains in the ∼900-nucleotide (nt) VP1 region (8). This definition follows from the high rate of nucleotide sequence evolution in poliovirus (∼1% per year) (9) and the normal period of poliovirus excretion of <3 months (10). VDPVs are distributed into three categories: (i) circulating VDPVs (cVDPVs) associated with outbreaks in settings of low OPV coverage (8, 11, 12), (ii) immunodeficiency-associated VDPVs (iVDPVs) from prolonged infections of persons with primary immunodeficiencies (8, 11, 13–16), and (iii) ambiguous VDPVs (aVDPVs) isolated from immunocompetent persons or the environment (8, 11, 12). Environmental aVDPVs in countries with high poliovirus vaccine coverage may signal latent chronic infections, but none of the infected persons have been identified so far (3, 8, 17–20).
The differing genetic properties of cVDPVs and iVDPVs facilitate predicting the likely sources of many aVDPVs (11, 12). Most cVDPVs are vaccine/nonvaccine recombinants with limited substitution in the neutralizing antigenic (NAg) sites (8, 12, 21). In contrast, most iVDPV isolates have nonrecombinant or vaccine/vaccine recombinant genomes, multiple substitutions in the NAg sites (8, 13, 14, 16), and numerous mixed-base positions (reflecting divergence into separate lineages during prolonged infections) (13, 14, 22–24).
In 2002, a highly evolved type 3 VDPV (VDPV3) was isolated from Tallinn sewage by the Central Laboratory of Virology, Estonia (3). A related VDPV3 was again isolated from Tallinn sewage in 2008, along with highly evolved VDPV2s in 2008, 2009, and 2010 (Tables 1 and 2). Sequence analysis (3) performed at the National Institute for Health and Welfare, Finland, showed that all VDPV isolates differed from their parental OPV strains in P1/capsid region nucleotides (∼2,550) by 13.5 to 15.1% (VDPV2) and 12.6 to 14.9% (VDPV3) (Tables 1 and 2). The Estonian VDPVs of each serotype formed unique genetic groupings distinct from recent wild poliovirus type 2 (WPV2) (see Fig. S1A in the supplemental material) and WPV3 (see Fig. S1B) genotypes and environmental VDPVs from other countries (see Fig. S1) (20). P1/capsid region sequences of the four VDPV2 isolates were up to 10.0% divergent from each other (Table 1), consistent with the emergence of separate VDPV2 lineages.
Table 1.
Nucleotide differences in P1/capsid region among the Estonian VDPV2 sewage isolates
| Designation | VDPV2 |
Pairwise nt difference P1/capsid regiona (%) |
|||||
|---|---|---|---|---|---|---|---|
| Virusb | Collection date (day/mo/yr) | S2 | 1 | 2 | 3 | 4 | |
| S2 | Sabin 2 | 2.3 | 2.5 | 2.4 | 2.7 | ||
| 1 | VDPV2/EST/Env2008 | 25/9/2008 | 13.5 | 1.3 | 1.3 | 1.3 | |
| 2 | VDPV2/EST/Env2009c | 16/11/2009 | 15.3 | 9.3 | 0.7 | 0.5 | |
| 3 | VDPV2/EST/Env2010-1 | 15/3/2010 | 14.6 | 9.5 | 5.8 | 0.9 | |
| 4 | VDPV2/EST/Env2010-2 | 25/11/2010 | 15.1 | 10.0 | 3.2 | 6.8 | |
Table 2.
Nucleotide differences in P1/capsid region among the Estonian VDPV3 sewage isolates
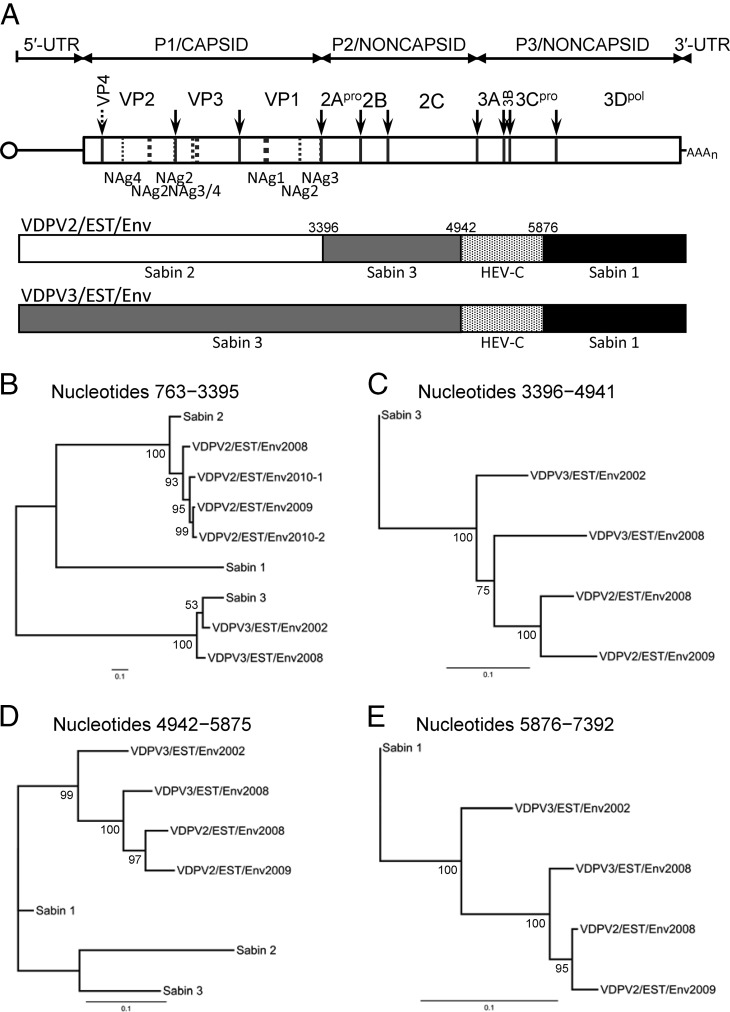
The Estonian VDPV isolates had mosaic genomes, with shared P2 and P3/noncapsid region recombination sites (Fig. 1A). The approximate sites of recombination, obscured in alignments of total nucleotide substitutions (3) by the high mutational background (see Fig. S2B and D, upper alignments, in the supplemental material), could be visualized in alignments showing only differences in the more slowly accumulating transversions (9) (see Fig. S2B and D, lower alignments), revealing a Sabin 3/human enterovirus (Sab3/HEV) site after nucleotide position 4941 and an HEV/Sab1 site after nucleotide position 5875 (see Fig. S2C, E, and G). Although our assignments were supported by analyses using the recombination detection program DualBrothers (25, 26) (see Fig. S3), we could not localize recombination sites with greater precision because of residual transversions and because the sequences of the parental HEV donor are unknown.
Fig 1.
Shared noncapsid region recombination sites among Estonian VDPV2 and VDPV3 sewage isolates. (A) Schematic of the poliovirus genome. The single ORF is indicated by a rectangle flanked by the 5′- and 3′-untranslated regions (UTRs); the small protein VPg, covalently attached to the 5′ end, is represented by a circle. Cleavage sites for processing of the polyprotein are indicated by arrows. Dashed lines symbolize virion surface loops forming neutralizing antigenic (NAg) sites 1, 2, 3, and 4. Mosaic structures of VDPV genomes are symbolized by differential shading. (B to E) Maximum likelihood trees (34) (http://www.geneious.com) summarizing sequence relationships among Estonian VDPV2 and VDPV3 isolates and the parental Sabin OPV strain sequences for genomic intervals approximately corresponding to different recombination blocks. (B) VDPV2 and VDPV3 P1/capsid region sequences (nt 763 to 3395) compared to the homologous Sabin strain sequences. (C) VDPV2 and VDPV3 sequences (nt 3396 to 4941) rooted to the homologous Sabin 3 sequences. (D) VDPV2 and VDPV3 sequences (nt 4942 to 5875) compared to the homologous Sabin strain sequences. (E) VDPV2 and VDPV3 sequences (nt 5876 to 7392) rooted to homologous Sabin 1 sequences. Nucleotide positions are numbered according to Toyoda et al. (35). Bootstrap values were obtained after 1,000 replicates and are shown at nodes of consensus trees.
The finding that all Estonian VDPVs shared common recombination sites is strong evidence of derivation from a chronically infected person fed trivalent OPV (tOPV) many years earlier. We estimated the date of the initiating tOPV dose from the rate of accumulation into the P1/capsid region of total nucleotide substitutions (KT), total synonymous substitutions (K′S), and synonymous transversions (B′S) (9) (Table 3). Mean rates of substitution into the VDPV2 P1/capsid region were estimated at 1.47 × 10−2 nt substitutions/site/year (KT), 1.78 × 10−2 nt substitutions/site/year (K′S), and 1.62 × 10−3 nt substitutions/site/year (B′S) (Table 3). Using these rate values, the total age of the most recent (25 November 2010) VDPV2 isolate was estimated at 18.5 (KT), 12.9 (K′S), and 17.1 years (B′S), with a mean of 16.2 years. If the actual KT evolution rate was similar to the rate (1.1 × 10−2 nt substitutions/site/year) calculated from more robust data sets (9, 21), then the age of the most recent isolate would be estimated at 21.9 years (date of the initiating tOPV dose, ∼1989) (Table 3). When the KT evolution rate was assumed to be 1.1 × 10−2 nt substitutions/site/year, the estimated age of the December 2009 VDPV3 isolate was 23.3 years (date of the initiating tOPV dose, ∼1986) (Table 3). Although each of these estimates has wide confidence intervals, it is clear that the VDPVs had been replicating for ≥15 years.
Table 3.
Bayesian Markov chain Monte Carlo estimates of the date of the initiating tOPV dose
| VDPV category | Substitution category | Mean substitution ratea (×10−2) | Substitution rate (95% HPDb) | Mean total age (yr) | Age in yr (95% HPD) | Mean estimate for year of initiating tOPV dosec |
|---|---|---|---|---|---|---|
| VDPV2 | KTd | 1.47 | 0.65–2.28 | 18.5 | 9.7–29.5 | 1992 |
| VDPV2 | K′eS | 1.78 | 1.07–2.52 | 12.9 | 7.8–18.6 | 1998 |
| VDPV2 | B′fS | 0.16 | 0.05–0.29 | 17.1 | 6.9–38.5 | 1994 |
| VDPV2 | KT(1.1)g | [1.10] | 21.9 | 16.1–27.1 | 1989 | |
| VDPV3 | KT(1.1) | [1.10] | 23.3 | 15.8–29.8 | 1986 |
Expressed as nucleotide substitutions/site/year for each category of substitution in the P1/capsid region. Substitution categories were normalized to total sites as described previously (9). Estimates from the data set are shown without brackets.
HPD, highest posterior density.
Rounded to the nearest beginning of the year.
KT, estimated total nucleotide substitutions.
K′S, estimated synonymous substitutions normalized to total sites.
B′S, estimated synonymous transversions normalized to total sites.
KT(1.1) assumes a rate of total nucleotide substitution of 1.1 × 10−2 nt substitutions/site/year.
The intertypic vaccine/vaccine recombinants emerged early in the infection, as is typically observed when individuals are fed tOPV (27). The mosaic structures of the noncapsid region may change over the 4- to 8-week period of excretion with further genetic exchanges among the OPV strains (27) but subsequently stabilize, and intertypic recombination sites remain conserved in highly divergent VDPV recombinants (3, 18, 24). P1/capsid region sequences of the Estonian VDPVs clustered by serotype on maximum likelihood trees, consistent with a pattern of little or no intertypic P1/capsid region recombination (Fig. 1B). In contrast, the Sabin 3-derived (nt 3396 to 4941) (Fig. 1C), HEV-derived (nt 4942 to 5875) (Fig. 1D), and Sabin 1-derived (nt 5876 to 7392) (Fig. 1E) noncapsid region sequences of the VDPV isolates did not cluster by serotype. Tree topologies in the noncapsid region revealed an ancestral node common to all VDPV isolates (Fig. 1C to E). The extent of substitution in the Sabin strain-derived intervals is also consistent with ≥15 years of replication. The nodes connecting VDPV3/EST/Env2008, VDPV2/EST/Env2008, and VDPV2/EST/Env2009 are shifted to the right for the Sabin 3-derived interval compared to the downstream Sabin 1-derived interval, suggesting more frequent genetic exchanges in the P3/noncapsid region than in the P2/noncapsid region, as would be expected if noncapsid region recombination frequencies are roughly proportional to the physical distance from the P1/capsid region.
As with other VDPVs (12, 14, 16, 18, 21, 24), the major determinants of the attenuated and temperature-sensitive phenotypes in Sabin 2 (A481→G and VP1-Ile143→Thr) (28, 29) had reverted in all VDPV2 isolates, and those of Sabin 3 (U472→C and VP3-Phe091→Ser) (30) had reverted in the VDPV3 isolates.
Four major NAg sites have been described for the poliovirus capsid: NAg-1, NAg-2, NAg-3, and NAg-4 (31). Among the four NAg sites, amino acid replacements had accumulated in all but NAg-3 in the VDPV2 isolates and in all but NAg-4 in the VDPV3 isolates (see Fig. S4 in the supplemental material). Each isolate had a unique NAg sequence, and the amino acid at position VP2-164 (NAg-2) was deleted from VDPV2/EST/Env-2009.
The Tallinn sewage isolates had properties typical of iVDPVs excreted by a chronically infected individual with a primary immunodeficiency, most likely common variable immunodeficiency (CVID) (3, 32). Unlike most individuals with iVDPV infections who either clear their infections or die within a year from complications of their immunodeficiency (8, 33), infected individuals with CVID may excrete iVDPVs for >5 years (13, 15, 16, 22). Some become paralyzed many years after the initial exposure to OPV (13, 16, 22), but others have remained asymptomatic (15). Consequently, environmental surveillance becomes a powerful tool for the detection of chronic, asymptomatic iVDPV infections (4). Highly divergent VDPVs resembling iVDPVs have also been found in sewage in Israel (VDPV2) (18), Slovakia (VDPV2) (19), and Finland (VDPV1, VDPV2, and VDPV3) (20). It is likely that high rates of poliovirus vaccine coverage, coupled with high levels of hygiene and sanitation, have limited the spread of the presumed iVDPVs in these developed countries. The presence of persons in the community with prolonged and unrecognized VDPV infections underscores the importance of maintaining high levels of population immunity to poliovirus years after all wild polioviruses have been eradicated.
Nucleotide sequence accession numbers.
The sequences determined in this study have been deposited in GenBank under accession numbers KC784367 to KC784373.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the World Health Organization (18-TSA-005).
The excellent technical assistance of Päivi Hirttiö, Alena Kaijalainen, Elisa Lamminsalo, Marja-Liisa Ollonen, Eija Penttilä, Mervi Eskelinen, and Johanna Rintamäki are greatly appreciated.
Footnotes
Published ahead of print 18 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01174-13.
REFERENCES
- 1.Cockburn WC, Drozdov SG. 1970. Poliomyelitis in the world. Bull. World Health Organ. 42:405–417 [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention 2013. Progress toward eradication of polio–worldwide, January 2011–March 2013. Morb. Mortal. Wkly. Rep. 62:335–338 [PMC free article] [PubMed] [Google Scholar]
- 3.Blomqvist S, Savolainen C, Laine P, Hirttio P, Lamminsalo E, Penttila E, Joks S, Roivainen M, Hovi T. 2004. Characterization of a highly evolved vaccine-derived poliovirus type 3 isolated from sewage in Estonia. J. Virol. 78:4876–4883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hovi T, Shulman LM, van der Avoort H, Deshpande J, Roivainen M, de Gourville EM. 2012. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol. Infect. 140:1–13 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention 2013. Evaluating surveillance indicators supporting the Global Polio Eradication Initiative, 2011–2012. Morb. Mortal. Wkly. Rep. 62:270–274 [PMC free article] [PubMed] [Google Scholar]
- 6.Minor PD, Almond JW. 2002. Poliovirus vaccines: molecular biology and immune response, p 381–390 In Semler BL, Wimmer E. (ed), Molecular biology of picornaviruses. ASM Press, Washington, DC [Google Scholar]
- 7.Sutter RW, Kew OM, Cochi SL, Aylward RB. 2013. Poliovirus vaccine–live, p 598–645 In Plotkin SA, Orenstein WA, Offit PA. (ed), Vaccines, 6th ed. W. B. Saunders, London, United Kingdom [Google Scholar]
- 8.Centers for Disease Control and Prevention 2012. Update on vaccine-derived polioviruses–worldwide, April 2011–June 2012. Morb. Mortal. Wkly. Rep. 61:741–746 [PubMed] [Google Scholar]
- 9.Jorba J, Campagnoli R, De L, Kew O. 2008. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J. Virol. 82:4429–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander JP, Jr, Gary HE, Jr, Pallansch MA. 1997. Duration of poliovirus excretion and its implications for acute flaccid paralysis surveillance: a review of the literature. J. Infect. Dis. 175:S176–S182 [DOI] [PubMed] [Google Scholar]
- 11.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. 2005. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 59:587–635 [DOI] [PubMed] [Google Scholar]
- 12.Joffret ML, Jégouic S, Bessaud M, Balanant J, Tran C, Caro V, Holmblat B, Razafindratsimandresy R, Reynes J-M, Rakoto-Andrianarivelo M, Delpeyroux F. 2012. Common and diverse features of co-circulating type 2 and 3 recombinant vaccine-derived polioviruses isolated from patients with poliomyelitis and healthy children. J. Infect. Dis. 205:1363–1373 [DOI] [PubMed] [Google Scholar]
- 13.Kew OM, Sutter RW, Nottay B, McDonough M, Prevots DR, Quick L, Pallansch M. 1998. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J. Clin. Microbiol. 36:2893–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martín J, Dunn G, Hull R, Patel V, Minor PD. 2000. Evolution of the Sabin strain of type 3 poliovirus in an immunodeficient patient during the entire 637-day period of virus excretion. J. Virol. 74:3001–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martín J. 2006. Vaccine-derived poliovirus from long term excretors and the end game of polio eradication. Biologicals 34:117–122 [DOI] [PubMed] [Google Scholar]
- 16.DeVries AS, Harper J, Murray A, Lexau C, Bahta L, Christensen J, Cebelinski E, Fuller S, Kline S, Wallace GS, Shaw JH, Burns CC, Lynfield R. 2011. Vaccine-derived poliomyelitis 12 years after infection in Minnesota. N. Engl. J. Med. 364:2316–2323 [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention 2011. Update on vaccine-derived polioviruses–worldwide, July 2009–March 2011. Morb. Mortal. Wkly. Rep. 60:846–850 [PubMed] [Google Scholar]
- 18.Shulman LM, Manor Y, Sofer D, Handsher R, Swartz T, Delpeyroux F, Mendelson E. 2006. Neurovirulent vaccine-derived polioviruses in sewage from highly immune populations. PLoS One 1:e69. 10.1371/journal.pone.0000069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cernáková B, Sobotová Z, Rovný I, Bláhova S, Roivainen M, Hovi T. 2005. Isolation of vaccine-derived polioviruses in the Slovak Republic. Eur. J. Clin. Microbiol. Infect. Dis. 24:438–439 [DOI] [PubMed] [Google Scholar]
- 20.Roivainen M, Blomqvist S, Al-Hello H, Paananen A, Delpeyroux F, Kuusi M, Hovi T. 2010. Highly divergent neurovirulent vaccine-derived polioviruses of all three serotypes are recurrently detected in Finnish sewage. Euro Surveill. 15:pii/19566. [PubMed] [Google Scholar]
- 21.Burns C, Shaw J, Jorba J, Bukbuk D, Adu F, Gumede N, Iber J, Chen Q, Vincent A, Chenoweth P, Henderson E, Wannemeuhler K, Campagnoli R, Pate MA, Abanida E, Gasasira A, Shimizu H, Williams AJ, Kilpatrick D, Wassilak S, Tomori O, Pallansch M, Kew O. 2013. Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria. J. Virol. 87:4907–4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellmunt A, May G, Zell R, Pring-Akerblom P, Verhagen W, Heim A. 1999. Evolution of poliovirus type I during 5.5 years of prolonged enteral replication in an immunodeficient patient. Virology 265:178–184 [DOI] [PubMed] [Google Scholar]
- 23.Yang C-F, Chen H-Y, Jorba J, Sun H-C, Yang S-J, Lee H-C, Huang Y-C, Lin T-Y, Chen P-J, Shimizu H, Nishimura Y, Utama A, Pallansch M, Miyamura T, Kew O, Yang J-Y. 2005. Intratypic recombination among lineages of type 1 vaccine-derived poliovirus emerging during chronic infection of an immunodeficient patient. J. Virol. 79:12623–12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahmahmoodi S, Parvaneh N, Burns C, Asghar H, Mamishi S, Tabatabaie H, Chen Q, Teimourian S, Gooya MM, Esteghamati A-R, Mousavi T, Yousefi M, Farrokhi K, Mashlool M, Kew O, Nategh R. 2008. Isolation of a type 3 vaccine-derived poliovirus (VDPV) from an Iranian child with X-linked agammaglobulinemia. Virus Res. 137:168–172 [DOI] [PubMed] [Google Scholar]
- 25.Minin VN, Dorman KS, Fang F, Suchard MA. 2005. Dual multiple change-point model leads to more accurate recombination detection. Bioinformatics 21:3034–3042 [DOI] [PubMed] [Google Scholar]
- 26.Suchard MA, Weiss RE, Dorman KS, Sinsheimer JS. 2003. Inferring spatial phylogenetic variation along nucleotide sequences: a multiple changepoint model. J. Am. Stat. Assoc. 98:427–437 [Google Scholar]
- 27.Cammack N, Phillips A, Dunn G, Patel V, Minor PD. 1988. Intertypic genomic rearrangements of poliovirus strains in vaccinees. Virology 167:507–514 [PubMed] [Google Scholar]
- 28.Macadam AJ, Pollard SR, Ferguson G, Skuce R, Wood D, Almond JW, Minor PD. 1993. Genetic basis of attenuation of the Sabin type 2 vaccine strain of poliovirus in primates. Virology 192:18–26 [DOI] [PubMed] [Google Scholar]
- 29.Ren R, Moss EG, Racaniello VR. 1991. Identification of two determinants that attenuate vaccine-related type 2 poliovirus. J. Virol. 65:1377–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westrop GD, Wareham KA, Evans DM, Dunn G, Minor PD, Magrath DI, Taffs F, Marsden S, Skinner MA, Schild GC, Almond JW. 1989. Genetic basis of attenuation of the Sabin type 3 oral poliovirus vaccine. J. Virol. 63:1338–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minor PD. 1990. Antigenic structure of picornaviruses. Curr. Top. Microbiol. Immunol. 161:121–154 [DOI] [PubMed] [Google Scholar]
- 32.Yong PFK, Thaventhiran JED, Grimbacher B. 2011. “A rose is a rose is a rose,” but CVID is not CVID common variable immune deficiency (CVID), what do we know in 2011? Adv. Immunol. 111:47–107 [DOI] [PubMed] [Google Scholar]
- 33.Khetsuriani N, Prevots DR, Quick L, Elder ME, Pallansch M, Kew O, Sutter RW. 2003. Persistence of vaccine-derived polioviruses among immunodeficient persons with vaccine-associated paralytic poliomyelitis. J. Infect. Dis. 188:1845–1852 [DOI] [PubMed] [Google Scholar]
- 34.Swofford DL. 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, MA [Google Scholar]
- 35.Toyoda H, Kohara M, Kataoka Y, Suganuma T, Omata T, Imura N, Nomoto A. 1984. Complete nucleotide sequences of all three poliovirus serotype genomes: implication for genetic relationship, gene function and antigenic determinants. J. Mol. Biol. 174:561–585 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.