Fig 4.
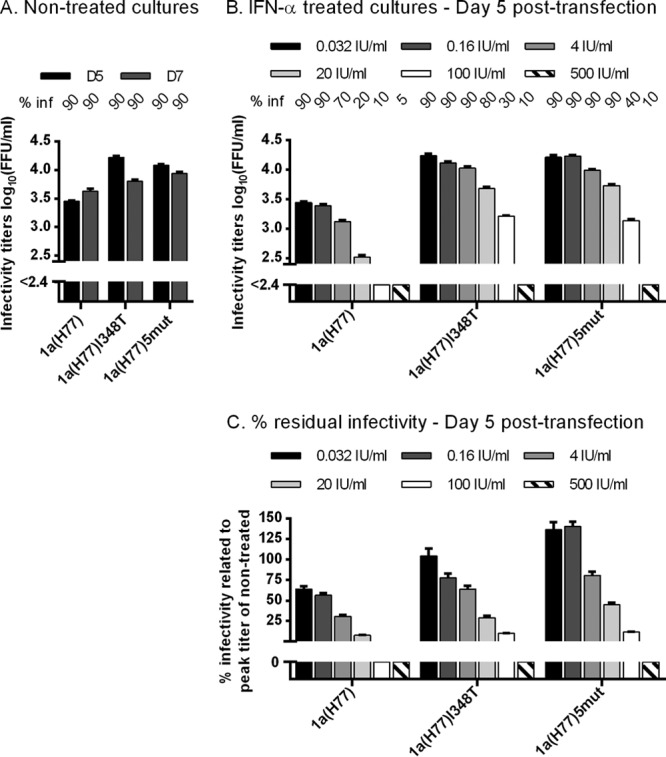
1a(H77) with the I348T substitution showed resistance to several concentrations of IFN-α. In vitro HCV RNA transcripts of the indicated recombinants were transfected into replicate Huh7.5 cultures. From day 1 posttransfection, cultures were split every second day. (A and B) The percentage of infected cultured cells (% inf) was monitored by immunostaining for HCV NS5A and is indicated above the graph at the indicated day posttransfection. Supernatant infectivity titers are means from 3 replicate determinations of 3 replicate cultures with SEM. The lower limit of detection in the experiments shown was up to 2.4 log10 FFU/ml, indicated by y axis breaks. (A) Cultures were monitored without IFN-α treatment until day 7 posttransfection. (B) Replicate cultures were treated with a 5-fold serial dilution of IFN-α2b (0.032 to 500 IU/ml, excluding 0.8 IU/ml); each concentration was tested in triplicate cultures. Treatment was initiated on day 1 posttransfection and administered each time the cells were split. Presented infectivity titers were from day 5 posttransfection; similar results were obtained at day 7. (C) Percent residual infectivity was determined by relating infectivity titers of treated cultures to peak infectivity titers of nontreated cultures infected with the same recombinant.
