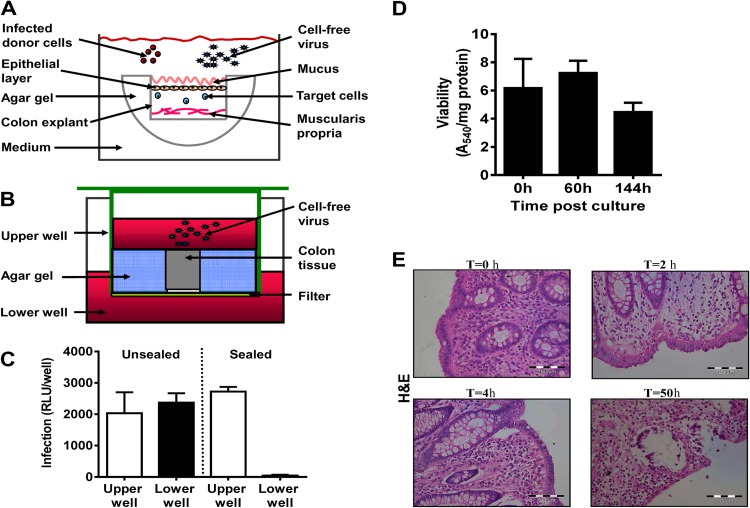
Fig 1.
Colon explant system. (A) Illustration of the explant sealing process. The sides and bottom of mucosal colon tissue pieces were sealed with 3% agar gel. The sealed tissue was placed in a well containing medium with the uncovered mucosal layer facing upward. Infected donor cells or cell-free virus was added to the medium to infect the sealed organ. (B) Validation of tissue explant sealing. Transwell plates were used to verify that free virus particles did not penetrate through the sides of the tissue explants. Sealed colon tissue was placed in the upper wells, and additional gel was used to seal the sides of the tissue blocks. Medium containing cell-free virus was added on top of the organ explants, while medium without virus was added to the lower wells. The wells were incubated at 37°C for 48 h. (C) To validate the sealing efficiency, replicating virus was quantified by collecting supernatants from the upper and lower wells, and virus titers were determined on TZM-bl reporter cells. An unsealed block exposed to cell-free virus in a transwell served as a control. Data reflect means ± SEMs (n = 3). RLU, relative light units. (D) Viability of sealed human colon tissues in organ culture. Human naive mucosal colon tissue specimens were cut into 4-mm circular pieces. Tissue pieces were sealed on the sides with agar gel and incubated for the indicated times. An MTT viability assay was carried out, and the results were corrected for the protein content of the human tissue as described in Materials and Methods. Data are means ± SEM (n = 5). (E) Histological sections (5 μm) were prepared from cultures of human colon tissues immediately following surgery and at times (T) of 2, 4, and 50 h after culture. The paraffin-embedded slides were stained with H&E.