Fig 5.
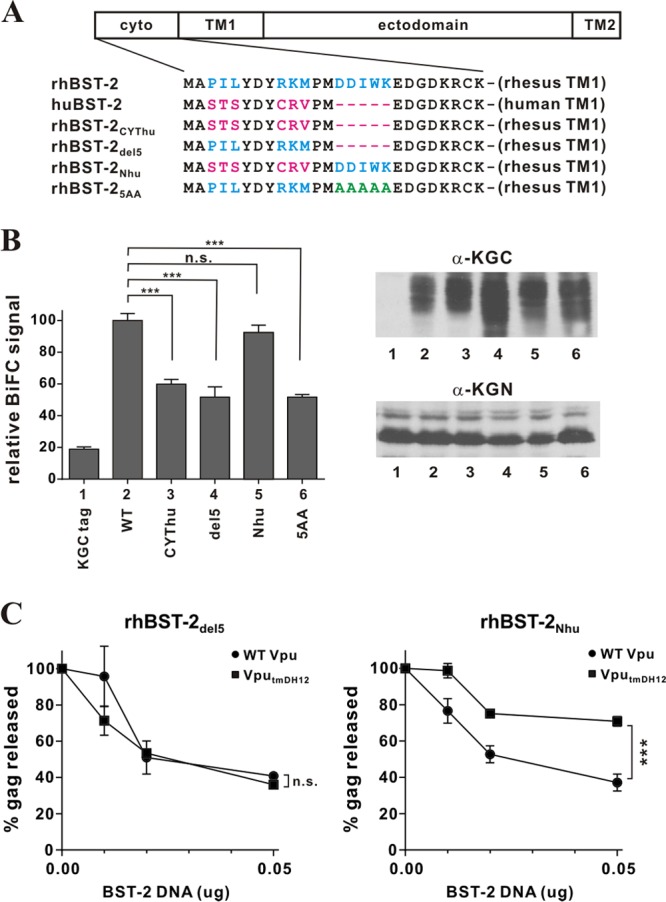
The 5-residue (G/D)DIWK motif in the cytoplasmic domain of rhesus BST-2 is important for interaction with Vpu. (A) Amino acid alignment of the cytoplasmic domains of the BST-2 variants used in this experiment. Identical sequences are shown in black. Residues in blue are rhesus BST-2-specific sequences; residues in red are human BST-2-specific sequences. The DDIWK-to-AAAAA substitution in rhBST-25AA is indicated in green. (B) (Left) The physical interaction of the rhBST-2 variants with NL4-3 Vpu was assessed by flow cytometry as described for Fig. 1B. The BiFC signal obtained for the interaction of Vpu and rhBST-2 (WT) (bar 2) was defined as 100%. ***, P < 0.01; n.s., P > 0.05. (Right) The expression of the KGC-tagged BST-2 variants and KGN-tagged Vpu was assessed by immunoblotting as described for Fig. 1B. The protein band corresponding to the KGC tag alone has a faster mobility and is not visible here. (C) The functional antagonism of rhesus BST-2 variants by Vpu was assessed in a virus release assay by metabolic labeling as described for Fig. 3C. Circles, NL4-3 (WT); squares, NL4-3 VputmDH12. Data are means ± standard errors of the means from three independent experiments. Statistical significance was determined by two-way ANOVA. ***, P < 0.01; n.s., P > 0.05.
