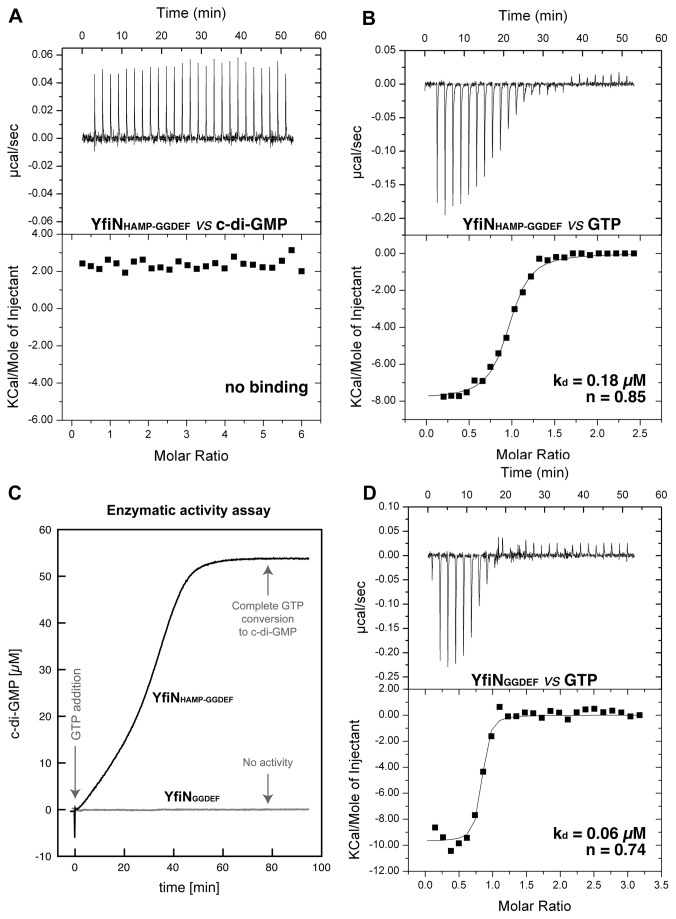
Figure 4. Binding affinity for nucleotides and enzymatic activity of YfiNHAMP-GGDEF and YfiNGGDEF.
For all ITC experiments upper panels show the Raw ITC data, while lower panels show the integrated peak areas (black square) fitted with the one-binding-site model of ORIGIN provided by MicroCal (continuous lines). Derived thermodynamic parameters are listed in Table 2 A) Microcalorimetric titration of 3 μM YfiNHAMP-GGDEF with c-di-GMP (90 μM in the syringe). No binding was observed either in the presence of CaCl2 or in the presence of MgCl2/MnCl2 (data not shown). No thermodynamic parameters were derived. B) Microcalorimetric titrations of 14 μM enzyme solution with GTP (170 μM in the syringe). The thermodynamic profile indicates that the interaction of YfiNHAMP-GGDEF with GTP presents favorable binding enthalpy and entropy, which suggests that hydrogen bonding and hydrophobic interactions are mainly involved in the binding event, rather than conformational changes. C) Cyclase activity of 10 µM YfiNHAMP-GGDEF or YfiNGGDEF assayed in real time by circular dichroism spectroscopy after addition of 100 µM GTP. For YfiNHAMP-GGDEF (Black) The final c-di-GMP concentration corresponds to complete conversion of 100 µM GTP, whilst for YfiNGGDEF (grey) no product is detected even if the sample is allowed to react for 24 h (not shown). D) Microcalorimetric titrations of 11 μM YfiNGGDEF with GTP (170 μM in the syringe).