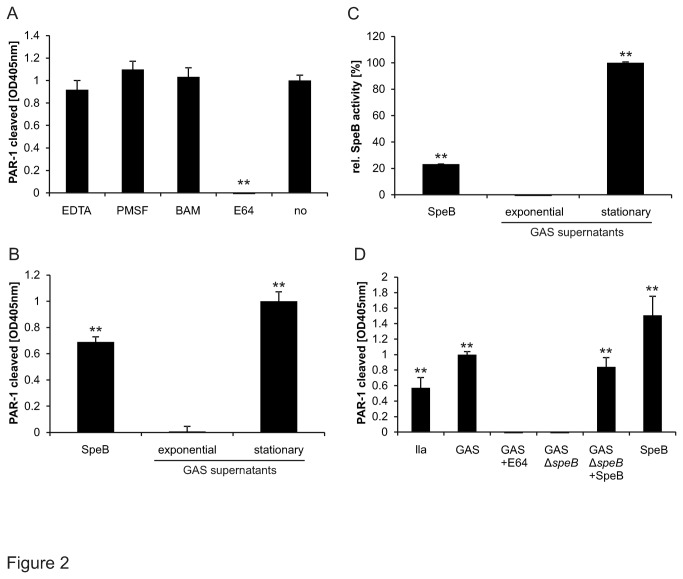
Figure 2. Streptococcal protease responsible for PAR-1 cleavage.
(A) GAS supernatants pre-incubated with either the metalloprotease inhibitor EDTA, the serine protein inhibitors PMSF and benzamidine (BAM), the cysteine protease inhibitor E64 or buffer were added onto 293T cells transiently over-expressing alkaline phosphatase-tagged PAR-1. Released alkaline phosphatase activity was quantified in the supernatants. (B) Cells as described in (A) were incubated with supernatants from exponential and stationary GAS to analyse their efficiency in cleaving AP-PAR-1 reporter constructs. (C) SpeB proteolytic activity was analysed by Bz-Pro-Phe-Arg-Nan cleavage in the exponential and stationary cultures GAS samples used in (B). Commercial SpeB (13.3 µg/ml) served as a positive control. (D) AP-PAR-1 reporter constructs were incubated with overnight cultures of non pre-treated wild type GAS, GAS preincubated with cysteine protease inhibitor E64 or the isogenic speB deficient GAS (GAS∆speB). In addition supernatants from GAS∆speB were complemented with commercial SpeB (200nM) and cleavage of AP-PAR-1 reporter construct was carried out. Thrombin (IIa, 1nM) served as positive control. Experiments were repeated at least 3 times with N=9, data presented as mean +/- SEM, *P<0.05, **P<0.01.