Abstract
Summary
Changes of the bone formation marker PINP correlated positively with improvements in vertebral strength in men with glucocorticoid-induced osteoporosis (GIO) who received 18-month treatment with teriparatide, but not with risedronate. These results support the use of PINP as a surrogate marker of bone strength in GIO patients treated with teriparatide.
Introduction
To investigate the correlations between biochemical markers of bone turnover and vertebral strength estimated by finite element analysis (FEA) in men with GIO.
Methods
A total of 92 men with GIO were included in an 18-month, randomized, open-label trial of teriparatide (20 μg/day, n = 45) and risedronate (35 mg/week, n = 47). High-resolution quantitative computed tomography images of the 12th thoracic vertebra obtained at baseline, 6 and 18 months were converted into digital nonlinear FE models and subjected to anterior bending, axial compression and torsion. Stiffness and strength were computed for each model and loading mode. Serum biochemical markers of bone formation (amino-terminal-propeptide of type I collagen [PINP]) and bone resorption (type I collagen cross-linked C-telopeptide degradation fragments [CTx]) were measured at baseline, 3 months, 6 months and 18 months. A mixed-model of repeated measures analysed changes from baseline and between-group differences. Spearman correlations assessed the relationship between changes from baseline of bone markers with FEA variables.
Results
PINP and CTx levels increased in the teriparatide group and decreased in the risedronate group. FEA-derived parameters increased in both groups, but were significantly higher at 18 months in the teriparatide group. Significant positive correlations were found between changes from baseline of PINP at 3, 6 and 18 months with changes in FE strength in the teriparatide-treated group, but not in the risedronate group.
Conclusions
Positive correlations between changes in a biochemical marker of bone formation and improvement of biomechanical properties support the use of PINP as a surrogate marker of bone strength in teriparatide-treated GIO patients.
Keywords: Biochemical markers of bone turnover, bone strength, finite element analysis, glucocorticoid-induced osteoporosis, male osteoporosis, teriparatide
Introduction
Biochemical markers of bone turnover (BTMs) are used as surrogate measures to evaluate the metabolic effect of drugs on bone turnover, and for predicting fracture risk in patients with osteoporosis [1, 2]. Changes in BTMs during anti-osteoporotic therapy depend on the cellular mechanism of action of the drug, magnitude of change in bone turnover rate, and route of administration [2]. Studies have found associations between treatment-related changes in BTMs with subsequent changes in bone mineral density (BMD), static and dynamic bone histomorphometric variables, and fracture outcomes during osteoporosis drug therapy [3–21]. However, these correlations are sometimes weak or non-significant, and can vary according to the BTMs measured, methodological limitations — including analytical variability — type of patients studied, and skeletal site assessed; they are also influenced by factors such as age, gender, use of prior osteoporosis medications and recent fracture [1, 2].
Bone strength, the maximum force a bone can bear, is the most important determinant of fracture risk and can be estimated in vivo in humans using finite element analysis (FEA) based on bone images obtained using quantitative computed tomography (QCT) [22–25]. Studies have shown an increase in vertebral strength during bisphosphonate and teriparatide treatment of postmenopausal women with osteoporosis [26–29] and in men with glucocorticoid-induced osteoporosis (GIO) [30].
The correlations between changes in BTMs and bone strength induced by pharmacological interventions have not previously been analysed in detail. Chevalier et al. [28] briefly reported a positive correlation between changes in bone strength and changes in the bone formation marker serum procollagen type I N-terminal propeptide (PINP) in postmenopausal women with osteoporosis treated with teriparatide after long-term exposure to bisphosphonates. However, the relationship between serum markers of bone turnover and bone strength during treatment with bisphosphonates and bone forming drugs in men with GIO has not been investigated before. GIO, the most common cause of secondary osteoporosis, is characterized by bone loss and impaired bone quality [31]. Chronic use of glucocorticoids (GC) leads to reduced bone formation via inhibition of differentiation, lifespan and function of osteoblasts and osteocytes [31–33]. This is reflected in decreased serum levels of bone formation markers in patients taking GC and, overall, a reduced bone turnover status in subjects with long-term GC treatment [34–36].
The aim of this predefined analysis of the EuroGIOPS trial (clinicaltrials.gov identifier: NCT00503399) was to examine the relationship between BTMs and bone strength estimated by high-resolution QCT (HRQCT)-based FEA at 6 and 18 months of therapy with teriparatide or risedronate in men with GIO. In particular, we determined the correlations between early changes in serum bone turnover markers with subsequent changes in bone strength under different loading conditions.
Methods
Study design
This 18-month, randomized, open-label, controlled study comparing the effects of teriparatide and risedronate in men with GIO was conducted at 16 centres in Germany, Greece, Italy, and Spain. The study design and baseline characteristics of the patients have been reported previously [30, 37]. Briefly, following a screening phase that lasted up to 6 weeks, patients attended a baseline visit at which they were randomized (1:1) to open-label treatment for 18 months with either teriparatide (20 μg once a day as a subcutaneous injection) or risedronate (35 mg once weekly orally as a tablet). Randomization was stratified by previous bisphosphonate use, and any previous osteoporosis treatment was discontinued during the screening phase before the baseline visit and for the duration of the study. During the study, all but one patient concomitantly received 1 g elemental calcium (as calcium carbonate alone or mixed with calcium lactogluconate), and 800–1,200 IU vitamin D/day. After randomization, patients attended clinic visits at approximately 3, 6, 12, and 18 months. The study was approved by the responsible institutional review boards at each centre and was conducted in accordance with the ethical standards of the Declaration of Helsinki and consistent with good clinical practice.
Participants
The patients enrolled in the study were men aged ≥25 years, ambulatory, with normal laboratory values for serum calcium, alkaline phosphatase, 25-hydroxyvitamin D and parathyroid hormone (PTH). They had a lumbar spine (L1 − L4), femoral neck, or total hip BMD T-score of at least 1.5 standard deviations (SDs) below the corresponding normal young adult men average BMD, and had at least two lumbar vertebrae without artefacts, fractures, or other abnormalities that would interfere with dual X-ray absorptiometry (DXA) or computed tomography (CT) assessments. Patients had received GC therapy at an average dose of at least 5.0 mg/day of prednisone or its equivalent for a minimum of 3 consecutive months immediately preceding the screening visit. Exclusion criteria included unresolved skeletal diseases other than GIO, presence of a spinal fracture in both T12 and L1, impaired renal function (creatinine clearance <30 ml/min/1.73 m2), abnormal thyroid function not corrected by therapy, history of symptomatic nephro- or urolithiasis in the year prior to randomization, malignant neoplasms in the 5 years prior to randomization, and any contraindication to therapy with teriparatide and risedronate. Patients were also excluded if they had taken intravenous bisphosphonates within 12 months prior to the screening visit, or strontium ranelate or fluoride at therapeutic doses (≥20 mg/day) for more than 3 months in the 2 years prior to randomization, or for more than a total of 2 years, or at any dosages within the 6 months prior to randomization. Previous treatment for any duration with calcitonin, oral bisphosphonates, or active vitamin D3 analogues that had been stopped prior to or at the randomization visit was allowed. All patients provided written informed consent.
Biochemical markers of bone turnover
Serum concentrations of two BTMs were measured at baseline and at 3, 6 and 18 months of treatment: (1) the bone formation marker PINP and (2) the bone resorption marker C-terminal cross-linked telopeptides of type I collagen (CTx). Fasting blood samples (10 ml) were collected in the morning, then serum samples were prepared and stored at −20 °C or lower at the study site for up to 4 months before being sent to a central laboratory (Covance, Geneva, Switzerland) for storage at −80 °C and processing. All samples from an individual were assayed in a single analytical batch. Serum intact PINP was measured by the Intact UniQ RIA assay (Orion Diagnostica, Espoo, Finland). This assay is not sensitive to the small molecular weight degradation products of the pro-peptide (cross-reaction only 1.2 %). The inter-assay (within day) analytical coefficient of variation (CV) was less than 3.1–8.2 % over the reference interval. Serum CTx was measured by the Serum Crosslaps® ELISA (Nordic Bioscience Diagnostics, Herlev, Denmark). The inter-assay CV was 5.4–11.4 %.
High-resolution quantitative CT and FEA
CT scans were performed at baseline and at 6 and 18 months of treatment. To optimize image quality serving as the input data for FE analyses, we used an HRQCT protocol rather than a standard QCT protocol with thicker slices and lower plane resolution. All HRQCT assessments performed in this study have been described elsewhere [30, 37], and are briefly summarized below.
A thin-slice spiral CT of the 12th thoracic vertebra (T12) was acquired using a scanner set at 120 kV and 360 mA s. If T12 was fractured, the HRQCT was performed on an intact L1 vertebra. Two images were reconstructed. The first one had a large field of view (FOV), included the patient and calibration phantom, and was used to calibrate the second image on which all analyses were carried out. The second image, with a smaller FOV size of 80 or 96 mm (pixel sizes of 0.156 or 0.188 mm) depending on the scanner type, included only the vertebra. In this latter image, the complete vertebral body was segmented using a semi-automatic algorithm. A template vertebra was registered in 3D to each scan and subsequently adapted to the individual anatomy by an active contour.
HRQCT-based FEA was used to estimate the effects of treatment on bone strength and stiffness at T12 using the technique described by Graeff et al. [38]. Digital finite element models were generated for each patient from the segmented HRQCT images at an isometric resolution of 1.3 mm. The superior and inferior endplates were embedded in a thin layer of polymethyl-methacrylate (PMMA) and the mineral density of each voxel/element was converted to bone volume fraction (BV/TV) with a calibration equation assuming a homogeneous tissue density. The bone tissue material behaviour was elastoplastic with damage; that is, irreversible strains develop and elastic modulus degrades with post-yield loading history. The model generation procedure and bone material properties have been described in detail by Chevalier et al. [39]. To account for a broad spectrum of physiological loading, the FEAs of each vertebral body included axial compression, anterior bending and axial torsion. The structural output variables computed by the FEAs were axial stiffness (kN/mm) and maximal load (kN) for axial compression, and angular stiffness (kN mm/rad) and maximal torque (kN mm) for anterior bending and axial torsion. A normalized strength in axial compression (N/mm2 = MPa) was also calculated as strength divided by the central cross-sectional area of the entire vertebral body.
All personnel in the radiology departments of the study sites were blinded to treatment assignment to reduce any potential bias from the open-label study design. Likewise, all scans were assessed centrally by radiology readers and engineers blinded to treatment assignment.
Statistical analysis
This was a pre-planned analysis of the EuroGIOPs clinical trial. All randomized patients who received at least one dose of study medication were included in the analyses. A mixed-model of repeated measures (MMRM) was used to analyse between-group differences and within- group changes by modelling the changes from baseline in BTM and FEA parameters. The model included terms for baseline value, treatment, visit, interaction between treatment and visit, age, baseline PINP, fracture within 12 months prior to study (yes/no), duration of bisphosphonate use, baseline GC dose, and cumulative GC doses before and during the study (fixed effects). Patients nested within treatment were included as random effects. Within the treatment groups, adjusted means obtained after controlling for the covariates (least square means [LS means]) with standard errors were derived at each of the follow-up visits. For differences between treatment groups, p values were derived and are presented in the results. The p values for the within group changes from baseline were derived and are indicated in the results when p < 0.05.
Within each treatment group, Spearman correlation coefficients with their associated two-sided p values, and residual mean square errors derived from linear regressions were both calculated for the absolute changes from baseline in BTMs at 3, 6 and 18 months with the changes from baseline in HRQCT-based FEA variables at 18 months. Scatter plots are presented and the regression lines are drawn to visualize relationships. The level of statistical significance was set to 5% and no multiplicity adjustments were performed. Data were analysed using SAS software© version 9.2.
Results
Patient disposition and baseline characteristics
Of the 174 male patients enrolled in the study, 92 were randomly assigned to receive treatment with teriparatide (n = 45) or risedronate (n = 47). Seventy-seven subjects (83.6 %) completed the 18-month treatment duration (teriparatide, n = 38; risedronate, n = 39), and 28 patients in each treatment group had HRQCT valid measurements.
The baseline demographic and clinical characteristics of the patients in the two treatment groups were similar and are reported in full elsewhere [30]. Mean age was 56.3 years (range 25–82 years) and 39.1% had at least one fracture prior to the study. Of the 92 patients, 31 (33.7 %) had a previous osteoporosis therapy, most commonly bisphosphonates (30 patients). All patients were on GC therapy prior to the study, mainly for musculoskeletal and connective tissue disorders (32.7 %), respiratory, thoracic and mediastinal disorders (23.6 %), or for gastrointestinal disorders (15.5 %). The median daily GC dose at baseline was 8.8 mg (interquartile range [IQR] 5.0–15.0 mg/day) and the median duration of prior GC therapy was 6.4 years (IQR 2.4–13.0 years).
Effects of treatment on bone turnover markers
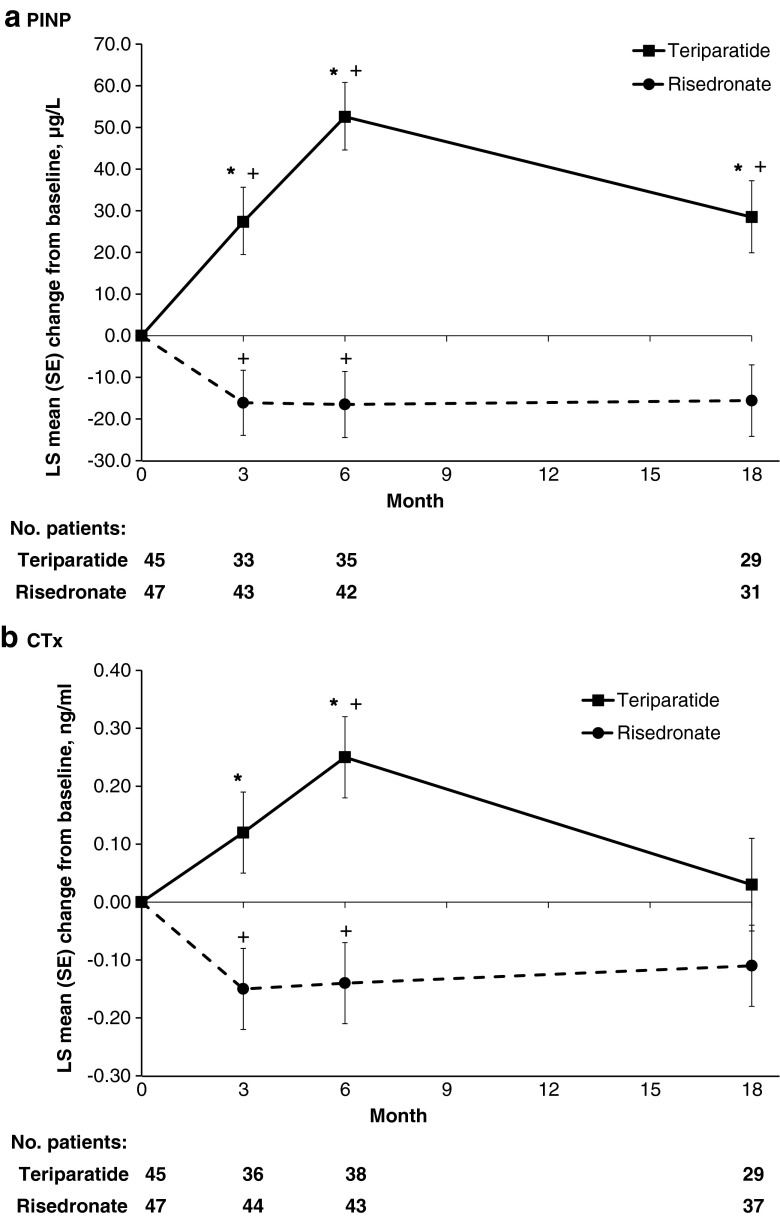
MMRM analysis revealed that the adjusted mean changes from baseline in PINP and CTx at 3, 6 and 18 months of therapy in the teriparatide and risedronate groups (Fig. 1) show significant differences between treatments at each of these time points (p < 0.001) with the exception of CTx at month 18 (p = 0.105).
Fig. 1.
Mean (SE) changes from baseline for the bone markers a PINP and b CTx at 3, 6 and 18 months in the teriparatide and risedronate treatment groups. *p < 0.001 for between-treatment comparisons, + p < 0.05 for change from baseline within groups. Mixed model repeated measures analysis of changes from baseline including fixed effects for treatment, visit and the interaction between treatment and visit, and random effects for patients nested within treatment, plus the following covariates: age, baseline PINP, fracture <12 months before study, duration of prior bisphosphonate use, screening GC dose, and cumulative GC dose prior to and during study. LS least squares, CTx type I collagen degradation fragments, PINP aminoterminal propeptide of type I procollagen, SE standard error
Changes in FEA variables during treatment
Table 1 summarizes the absolute values of the finite element strength variables under the different loading modes (anterior bending, axial compression, and axial torsion) and the results for axial strength after normalization by vertebral body size at baseline and at 6 and 18 months of treatment in the teriparatide and risedronate groups. There were increases from baseline during treatment in both groups. MMRM analysis showed that the increases in finite element strength and normalized axial compression strength at 18 months were significantly higher in the teriparatide group compared with the risedronate group (p ≤ 0.05). The between-treatment differences were not statistically significant at 6 months (Table 1). Similar results were observed for stiffness (data not shown).
Table 1.
Finite element strength in the different loading modes (anterior bending, axial compression, axial torsion) and normalized axial compression strength for the teriparatide and risedronate treatment groups
| Variable | Time (months) | Teriparatide | Risedronate | p value a | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||
| Finite element strength | ||||||
| Anterior bending (kN mm) | Baseline | 36 | 94.7 (41.8) | 36 | 96.2 (42.3) | – |
| 6 | 25 | 121.3 (49.9) | 32 | 113.5 (46.0) | 0.661 | |
| 18 | 29 | 140.2 (58.8)b | 31 | 112.8 (40.8) | 0.012 | |
| Axial compression (kN) | Baseline | 36 | 5.07 (2.33) | 37 | 4.90 (2.28) | – |
| 6 | 25 | 6.21 (2.87) | 33 | 5.81 (2.23) | 0.547 | |
| 18 | 31 | 7.08 (3.48)b | 31 | 5.95 (2.2) | 0.015 | |
| Axial torsion (kN mm) | Baseline | 36 | 48.4 (22.1) | 37 | 48.6 (21.2) | – |
| 6 | 25 | 62.4 (26.3) | 33 | 57.9 (20.9) | 0.548 | |
| 18 | 31 | 71.0 (31.8)b | 31 | 58.2 (19.2) | 0.005 | |
| Normalized axial compression strength (N/mm2) | ||||||
| Baseline | 36 | 4.50 (2.20) | 37 | 4.41 (2.16) | – | |
| 6 | 25 | 5.32 (2.71) | 33 | 5.25 (2.18) | 0.677 | |
| 18 | 31 | 6.13 (3.29)b | 31 | 5.38 (2.08) | 0.021 | |
a p value for between group comparison
bChange from baseline within groups (p < 0.05) from a mixed model repeated-measures analysis of changes from baseline including fixed effects for treatment, visit and the interaction between treatment and visit, and random effects for patients nested within treatment, plus the following covariates: age, baseline PINP, fracture <12 months before study, duration of prior bisphosphonate use, screening GC dose, and cumulative GC dose prior to and during study. MMRM sample sizes for changes from baseline to 6 months (n = 23), and to 18 months (n = 28) for Teriparatide; and baseline to 6 months (n = 28), and to 18 months (n = 28) for Risedronate
Correlations between changes in bone turnover markers and changes in FEA variables
Table 2 presents the Spearman correlation coefficients between the absolute changes from baseline of PINP at 3, 6 and 18 months and the absolute changes from baseline in FEA parameters at 18 months of therapy in the teriparatide and risedronate groups. Significant positive correlations between the change in PINP at 3, 6 and 18 months with the changes in finite element strength and stiffness in all loading modes at 18 months (anterior bending, axial compression, and axial torsion) and in the change in normalized axial compression strength were observed in the teriparatide group (r = 0.422 to r = 0.563). Based on PINP measurements at the three time points, the changes in anterior bending strength associated with teriparatide treatment could be estimated with residual mean square errors between 37.0 and 42.0 kN mm. Similarly, the changes in axial compression and axial torsion strengths could be estimated with residual mean square errors between 2.1 and 2.3 kN, and 17.9 and 20.7 kN mm, respectively. There were no significant correlations in the risedronate-treated group (Table 2). Figure 2 shows the absolute change correlations of PINP at 3, 6 and 18 months with finite element strength variables at 18 months in the teriparatide and risedronate groups.
Table 2.
Spearman correlation coefficients (r values) between the absolute changes in serum PINP and CTx at 3, 6, and 18 months and the absolute changes in FEA parameters at 18 months by treatment group
| Time (months) | Finite element strength | Finite element stiffness | Normalized axial compression | |||||
|---|---|---|---|---|---|---|---|---|
| Anterior bending | Axial compression | Axial torsion | Anterior bending | Axial compression | Axial torsion | |||
| PINP | ||||||||
| Teriparatide | Δ3 | 0.422* | 0.516** | 0.496* | 0.397 | 0.525* | 0.402 | 0.539** |
| (n = 23) | (n = 24) | (n = 24) | (n = 24) | (n = 24) | (n = 24) | (n = 24) | ||
| Δ6 | 0.486* | 0.560*** | 0.544*** | 0.477* | 0.550* | 0.472* | 0.563*** | |
| (n = 23) | (n = 25) | (n = 25) | (n = 25) | (n = 25) | (n = 25) | (n = 25) | ||
| Δ18 | 0.546* | 0.522* | 0.455* | 0.413 | 0.517* | 0.403 | 0.553** | |
| (n = 19) | (n = 21) | (n = 21) | (n = 21) | (n = 21) | (n = 21) | (n = 21) | ||
| Risedronate | Δ3 | −0.033 | 0.043 | 0.031 | −0.093 | 0.021 | −0.084 | 0.046 |
| (n = 27) | (n = 27) | (n = 27) | (n = 27) | (n = 27) | (n = 27) | (n = 27) | ||
| Δ6 | −0.023 | 0.006 | 0.028 | −0.048 | −0.005 | −0.046 | 0.001 | |
| (n = 29) | (n = 29) | (n = 29) | (n = 29) | (n = 29) | (n = 29) | (n = 29) | ||
| Δ18 | −0.141 | −0.213 | −0.239 | −0.316 | −0.297 | −0.358 | −0.195 | |
| (n = 23) | (n = 23) | (n = 23) | (n = 23) | (n = 23) | (n = 23) | (n = 23) | ||
| CTx | ||||||||
| Teriparatide | Δ3 | 0.353 | 0.380 | 0.350 | 0.321 | 0.383* | 0.331 | 0.399* |
| (n = 26) | (n = 27) | (n = 27) | (n = 27) | (n = 27) | (n = 27) | (n = 27) | ||
| Δ6 | 0.382 | 0.380* | 0.339 | 0.254 | 0.379* | 0.284 | 0.412* | |
| (n = 26) | (n = 28) | (n = 28) | (n = 28) | (n = 28) | (n = 28) | (n = 28) | ||
| Δ18 | 0.381 | 0.382 | 0.326 | 0.217 | 0.367 | 0.236 | 0.424 | |
| (n = 18) | (n = 20) | (n = 20) | (n = 20) | (n = 20) | (n = 20) | (n = 20) | ||
| Risedronate | Δ3 | −0.099 | −0.052 | −0.027 | −0.096 | −0.062 | −0.103 | −0.050 |
| (n = 29) | (n = 29) | (n = 29) | (n = 29) | (n = 29) | (n = 29) | (n = 29) | ||
| Δ6 | −0.070 | −0.015 | −0.003 | 0.006 | 0.003 | 0.005 | −0.029 | |
| (n = 30) | (n = 30) | (n = 30) | (n = 30) | (n = 30) | (n = 30) | (n = 30) | ||
| Δ18 | −0.118 | −0.225 | −0.202 | −0.198 | −0.248 | −0.220 | −0.214 | |
| (n = 28) | (n = 28) | (n = 28) | (n = 28) | (n = 28) | (n = 28) | (n = 28) | ||
Δ3, Δ6 and Δ18 respectively represent change from baseline in serum PINP/CTx at 3, 6 and 18 months versus changes from baseline in FEA parameters at 18 months.
FEA finite element analysis, PINP aminoterminal propeptide of type I procollagen, CTx cross-linked C-telopeptide of type I collagen
*p < 0.05; **p ≤ 0.01; ***p ≤ 0.005
Fig. 2.
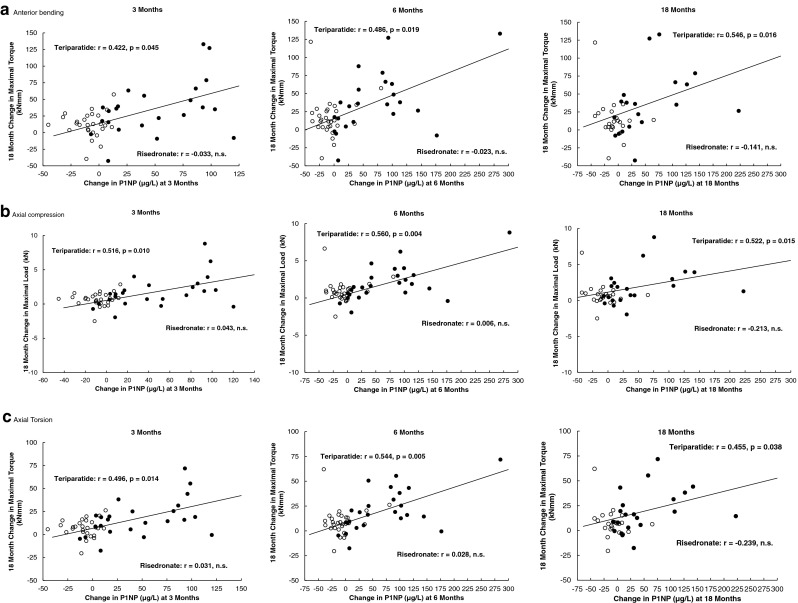
Scatter plots for the absolute change from baseline of PINP at 3, 6 and 18 months against the absolute change in finite element strength at 18 months of therapy with teriparatide (filled circles) or risedronate (open circles) for the three loading modes: a anterior bending, b axial compression, and c axial torsion. Each figure shows the trend line for correlations with p < 0.05 for teriparatide. r Spearman rank correlation coefficient
There were few significant correlations between absolute changes in serum CTx and absolute changes in FE strength variables in either treatment group (Table 2).
Discussion
Our study is the first to examine the relationship between changes in serum bone turnover markers and changes in FE-computed vertebral strength in men with GIO during osteoporosis drug therapy. We found a strong correlation between the increase in PINP, a bone formation marker, at 6 months and the subsequent increase in vertebral strength for all tested loading modes in the teriparatide-treated group, but not in the risedronate-treated group. Moreover, the analysis of the residual mean square errors indicates that the estimations of the changes in strength indices based on PINP changes in the teriparatide group were meaningful. This supports that PINP could be used as a surrogate marker of biomechanical indices in GIO patients treated with teriparatide, given the well-known correlation between FE-derived bone strength analysis and fractures [25, 40].
Our results complement previous findings in studies that have analysed the correlations between the bone marker response to teriparatide and other bone endpoints, such as BMD [4, 9, 13, 16, 18, 21, 41], histomorphometric variables [10, 42, 43] and spine strength [44] in patients with osteoporosis. In general, the strength of the correlations we have observed with FE analysis is numerically higher than with other bone parameters reported in teriparatide-treated subjects.
Chevalier et al. [28] previously reported a statistically significant correlation between the area under the curve PINP concentrations from baseline to 12 months and the change in FEA-estimated vertebral bone strength in 171 postmenopausal women with osteoporosis treated with teriparatide in the OPTAMISE study. Based on the square of the correlations, they showed that 19 % of the variation in the percentage change in maximal load can be explained by PINP changes after 12 months of treatment with teriparatide, while our equivalent analysis yields a maximum of 31% of the variation in the percentage of the axial compression strength after 18 months being explained by the PINP early changes. Besides the timing of the assessments, the two studies differ in patient population characteristics (all women in the OPTAMISE study received bisphosphonates prior to teriparatide for at least 2 years), and in the CT methods applied to evaluate the FE-derived strength; these differences may explain the differential results between the two studies. Additionally, the assay used in our study measures intact PINP, while investigators in the OPTAMISE trial used a different method that measures total PINP (i.e., including monomer and trimer).
Recently, there has been growing interest in the potential value of monitoring bone marker changes and bone strength to evaluate treatment response and fracture outcomes. Previous studies have shown an association between changes in bone turnover markers and fracture incidence/risk in postmenopausal women treated with antiresorptive therapies, including alendronate [7], risedronate [19, 45] and raloxifene [5, 6, 8], but not with strontium ranelate [46] or zoledronic acid [15]. Researchers from the EUROFORS trial reported the lack of a significant relationship between changes in biochemical markers and fracture risk in postmenopausal women treated with teriparatide [18]. However, these results should be interpreted with caution given the low number of subjects with incident fractures during the course of the study, and the lack of power to detect any potential correlations. Further studies are needed to define the role of biochemical markers as predictors of fracture outcomes during teriparatide therapy.
Studies have shown that, in general, there is an association between bone strength assessed by different types of QCT methods and fractures in men and women with osteoporosis [47–51]. Specifically, vertebral fractures are strongly associated with vertebral strength estimated using FE models in men older than 65 years [51] and in postmenopausal women [47]. In the baseline analysis of the EuroGIOPS study in men with GIO, all HRQCT-based FEA estimates of vertebral bone strength were significantly correlated with vertebral fracture status at baseline [37]. Additionally, trabecular BMD measured using QCT or HRQCT, but not BMD by DXA, was associated with vertebral fracture status [37]. Vertebral fractures in men have also been associated with bone strength estimated by QCT-based FEA at the hip [48] and at the distal radius and tibia [52].
A novel approach in our study was the analysis using three loading modes for vertebral bone strength, including axial torsion, which has not been examined before. We also accounted for bone size by normalizing bone strength with cross-sectional area of the entire vertebral body. All these measures of vertebral bone strength increased to a greater extent in the teriparatide group compared with the risedronate group, with no major differences depending on the loading mode, although the axial compression strength showed higher correlations with changes in PINP.
The observed increase in strength in axial compression in our study in the teriparatide-treated subjects (26.0 %) and in the risedronate group (4.2 %) [30] yielded similar results compared to previous studies of the effects of teriparatide and alendronate treatment on vertebral strength in postmenopausal women with osteoporosis, where Keaveny et al. [26] have shown increases in FE-assessed vertebral strength of 21 % with teriparatide versus 4 % with alendronate at 18 months, and Graeff et al. [27] have reported a 28 % increase in compressive and bending strength at 2 years of teriparatide treatment.
Similarly, the observed increases in PINP and CTx in the teriparatide-treated group are consistent with previous studies showing an increase in bone turnover markers during teriparatide therapy in men and women with osteoporosis [13, 18, 36, 42, 53–57]. Specifically, the maximum change from baseline in PINP and CTx was seen at 6 months; this was followed by a decrease in bone marker levels but, at 18 months, the level of PINP remained increased relative to baseline. This pattern of change in serum PINP levels has been observed in other studies of teriparatide-treated patients with GIO [36, 56], in postmenopausal women with osteoporosis [18, 42], and in men with osteoporosis [13]. Moreover, the absolute change from baseline in PINP at every time point in our study was well above the least significant change determined previously (10 μg/l) and used to monitor the early response to teriparatide treatment [21, 55].
Although our study has several important strengths, such as the prospective design in a group of patients with osteoporosis who have scarcely been evaluated in clinical trials, the application for the first time of novel HRQCT-based FE analysis in men with GIO, and a MMRM analysis adjusted for factors such as age, prior fracture, duration of prior bisphosphonate use and GC dose, it also has some limitations. These include that the analysis was restricted to only one vertebra (T12), but vertebral strength may vary along the spine. Second, the FE analysis assumes that bone tissue properties are constant for all patients during longitudinal treatment. However, since the patients involved in the study were GC users for several years, we do not expect a change in the local BMD–strength relationship in the course of the study. A hypothetical shift of the local BMD–strength relationship due to GC therapy throughout the study would influence neither the trends of the FE analysis nor the reported correlations. Other limitations of the study are that the duration of treatment was for 18 months only and the limited sample size. Longer treatment may offer even more pronounced advantages for both drugs. Although we only measured serum levels of PINP and CTx, these have recently been recommended as the reference markers of bone turnover to be used in clinical studies [1].
In conclusion, teriparatide at 20 μg/day demonstrated superior efficacy compared to risedronate 35 mg/week in the effects on biomechanical indices estimated by HRQCT-based FEA at the 12th thoracic vertebra in male patients with GIO. The changes from baseline in PINP revealed significant positive correlations with the changes in vertebral strength in all the loading modes at 18 months in the teriparatide group only. Changes in serum CTx showed fewer correlations. Serial spine QCT involves exposure to significant levels of radiation and considerable costs, which will limit its widespread use in normal clinical practice as an indicator of vertebral bone strength. Because bone strength estimated using this method was correlated significantly with serum levels of the bone formation marker PINP during teriparatide treatment, this suggests that monitoring of PINP may be clinically useful as a surrogate marker of biomechanical properties in GIO patients treated with teriparatide, but further studies with larger study populations and correlations with fracture outcomes are needed.
Acknowledgments
The authors thank all the patients who participated in the study. The authors also thank Beatriz Sanz (central study coordination) and Nadine L. McCann (central laboratory coordination) at Eli Lilly and Company for their support. Deirdre Elmhirst, Elmhirst Medical Writing Services, provided medical writing support. Funding was provided by Lilly Research Centre, Europe.
Conflicts of interest
The EuroGIOPS study was funded by Lilly Research Center, Europe (ClinicalTrials.gov identifier: NCT00503399). J.D. Ringe has received consulting fees or paid advisory boards from Amgen, Madaus, Merck, and Servier, and lecture fees from Leo, Eli Lilly, Novartis, Servier and Teva. N. Papaioannou has received research grants and/or consulting or speaking fees from Amgen, Eli Lilly and Servier. C-C. Glüer and P.K. Zysset have received honoraria and research support from Eli Lilly & Company. C. Niedhart has received honoraria from Eli Lilly & Company. A. Reisinger’s contribution was supported by Eli Lilly & Company. F. Marin, A. Gentzel, and H. Petto are employees of Eli Lilly & Company. All other coauthors have nothing to declare.
References
- 1.Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S, Trenti T, Wahl DA, Cooper C, Kanis JA, IOF–IFCC Bone Marker Standards Working Group Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 2.Szulc P. The role of bone turnover markers in monitoring treatment in postmenopausal osteoporosis. Clin Biochem. 2012;45:907–919. doi: 10.1016/j.clinbiochem.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Ravn P, Clemmesen B, Christiansen C. Biochemical markers can predict the response in bone mass during alendronate treatment in early postmenopausal women. Alendronate Osteoporosis Prevention Study Group. Bone. 1999;24:237–244. doi: 10.1016/S8756-3282(98)00183-5. [DOI] [PubMed] [Google Scholar]
- 4.Lane NE, Sanchez S, Genant HK, Jenkins DK, Arnaud CD. Short-term increases in bone turnover markers predict parathyroid hormone-induced spinal bone mineral density gains in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int. 2000;11:434–442. doi: 10.1007/s001980070111. [DOI] [PubMed] [Google Scholar]
- 5.Bjarnason NH, Sarkar S, Duong T, Mitlak B, Delmas PD, Christiansen C. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int. 2001;12:922–930. doi: 10.1007/s001980170020. [DOI] [PubMed] [Google Scholar]
- 6.Reginster JY, Sarkar S, Zegels B, Henrotin Y, Bruyere O, Agnusdei D, Collette J. Reduction in PINP, a marker of bone metabolism, with raloxifene treatment and its relationship with vertebral fracture risk. Bone. 2004;34:344–351. doi: 10.1016/j.bone.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, Thompson DE, Ewing SK, Delmas PD; Fracture Intervention Trial Study Group Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res. 2004;19:1250–1258. doi: 10.1359/JBMR.040512. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar S, Reginster JY, Crans GG, Diez-Perez A, Pinette KV, Delmas PD. Relationship between changes in biochemical markers of bone turnover and BMD to predict vertebral fracture risk. J Bone Miner Res. 2004;19:394–401. doi: 10.1359/JBMR.0301243. [DOI] [PubMed] [Google Scholar]
- 9.Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, Wagman RB. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005;20:962–970. doi: 10.1359/JBMR.050105. [DOI] [PubMed] [Google Scholar]
- 10.Dobnig H, Sipos A, Jiang Y, Fahrleitner-Pammer A, Ste-Marie LG, Gallagher JC, Pavo I, Wang J, Eriksen EF. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J Clin Endocrinol Metab. 2005;90:3970–3977. doi: 10.1210/jc.2003-1703. [DOI] [PubMed] [Google Scholar]
- 11.Greenspan SL, Resnick NM, Parker RA. Early changes in biochemical markers of bone turnover are associated with long-term changes in bone mineral density in elderly women on alendronate, hormone replacement therapy, or combination therapy: a three-year, double-blind, placebo-controlled, randomized clinical trial. J Clin Endocrinol Metab. 2005;90:2762–2767. doi: 10.1210/jc.2004-1091. [DOI] [PubMed] [Google Scholar]
- 12.Bauer DC, Garnero P, Bilezikian JP, Greenspan SL, Ensrud KE, Rosen CJ, Palermo L, Black DM. Short-term changes in bone turnover markers and bone mineral density response to parathyroid hormone in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2006;91:1370–1375. doi: 10.1210/jc.2005-1712. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein JS, Leder BZ, Burnett SM, Wyland JJ, Lee H, de la Paz AV, Gibson K, Neer RM. Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab. 2006;91:2882–2887. doi: 10.1210/jc.2006-0190. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs JW, de Nijs RN, Lems WF, Geusens PM, Laan RF, Huisman AM, Algra A, Buskens E, Hofbauer LC, Oostveen AC, Bruyn GA, Dijkmans BA, Bijlsma JW. Prevention of glucocorticoid induced osteoporosis with alendronate or alfacalcidol: relations of change in bone mineral density, bone markers, and calcium homeostasis. J Rheumatol. 2007;34:1051–1057. [PubMed] [Google Scholar]
- 15.Delmas PD, Munoz F, Black DM, Cosman F, Boonen S, Watts NB, Kendler D, Eriksen EF, Mesenbrink PG, Eastell R; HORIZON-PFT Research Group Effects of yearly zoledronic acid 5 mg on bone turnover markers and relation of PINP with fracture reduction in postmenopausal women with osteoporosis. J Bone Miner Res. 2009;24:1544–1551. doi: 10.1359/jbmr.090310. [DOI] [PubMed] [Google Scholar]
- 16.Burshell AL, Möricke R, Correa-Rotter R, Chen P, Warner MR, Dalsky GP, Taylor KA, Krege JH. Correlations between biochemical markers of bone turnover and bone density responses in patients with glucocorticoid-induced osteoporosis treated with teriparatide or alendronate. Bone. 2010;46:935–939. doi: 10.1016/j.bone.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg MC, Silverman SL, Barr CE, Miller PD. The utility of changes in serum levels of C-terminal telopeptide of type I collagen in predicting patient response to oral monthly ibandronate therapy. J Clin Densitom. 2010;13:181–189. doi: 10.1016/j.jocd.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Blumsohn A, Marin F, Nickelsen T, Brixen K, Sigurdsson G, González de la Vera J, Boonen S, Liu-Léage S, Barker C, Eastell R; EUROFORS Study Group Early changes in biochemical markers of bone turnover and their relationship with bone mineral density changes after 24 months of treatment with teriparatide. Osteoporos Int. 2011;22:1935–1946. doi: 10.1007/s00198-010-1379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eastell R, Vrijens B, Cahall DL, Ringe JD, Garnero P, Watts NB. Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J Bone Miner Res. 2011;26:1662–1669. doi: 10.1002/jbmr.342. [DOI] [PubMed] [Google Scholar]
- 20.Eastell R, Christiansen C, Grauer A, Kutilek S, Libanati C, McClung MR, Reid IR, Resch H, Siris E, Uebelhart D, Wang A, Weryha G, Cummings SR. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res. 2011;26:530–537. doi: 10.1002/jbmr.251. [DOI] [PubMed] [Google Scholar]
- 21.Tsujimoto M, Chen P, Miyauchi A, Sowa H, Krege JH. PINP as an aid for monitoring patients treated with teriparatide. Bone. 2011;48:793–803. doi: 10.1016/j.bone.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Faulkner KG, Cann CE, Hasegawa BH. Effect of bone distribution on vertebral strength: assessment with patient-specific nonlinear finite element analysis. Radiology. 1991;179:669–674. doi: 10.1148/radiology.179.3.2027972. [DOI] [PubMed] [Google Scholar]
- 23.Crawford RP, Cann CE, Keaveny TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003;33:744–750. doi: 10.1016/S8756-3282(03)00210-2. [DOI] [PubMed] [Google Scholar]
- 24.Griffith JF, Genant HK. New imaging modalities in bone. Curr Rheumatol Rep. 2011;13:241–250. doi: 10.1007/s11926-011-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dall’Ara E, Pahr D, Varga P, Kainberger F, Zysset P. QCT-based finite element models predict human vertebral strength in vitro significantly better than simulated DEXA. Osteoporos Int. 2012;23:563–572. doi: 10.1007/s00198-011-1568-3. [DOI] [PubMed] [Google Scholar]
- 26.Keaveny TM, Donley DW, Hoffmann PF, Mitlak BH, Glass EV, San Martin JA. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res. 2007;22:149–157. doi: 10.1359/jbmr.061011. [DOI] [PubMed] [Google Scholar]
- 27.Graeff C, Chevalier Y, Charlebois M, Varga P, Pahr D, Nickelsen TN, Morlock MM, Glüer CC, Zysset PK. Improvements in vertebral body strength under teriparatide treatment assessed in vivo by finite element analysis: results from the EUROFORS study. J Bone Miner Res. 2009;24:1672–1680. doi: 10.1359/jbmr.090416. [DOI] [PubMed] [Google Scholar]
- 28.Chevalier Y, Quek E, Borah B, Gross G, Stewart J, Lang T, Zysset P. Biomechanical effects of teriparatide in women with osteoporosis treated previously with alendronate and risedronate: results from quantitative computed tomography-based finite element analysis of the vertebral body. Bone. 2010;46:41–48. doi: 10.1016/j.bone.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 29.Keaveny TM, McClung MR, Wan X, Kopperdahl DL, Mitlak BH, Krohn K. Femoral strength in osteoporotic women treated with teriparatide or alendronate. Bone. 2012;50:165–170. doi: 10.1016/j.bone.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Gluer CC, Marin F, Ringe JD, Hawkins F, Moricke R, Papaioannu N, Farahmand P, Minisola S, Martinez G, Nolla J, Niedhart C, Guanabens N, Nuti R, Martin-Mola E, Thomasius F, Kapetanos G, Pena J, Graeff C, Petto H, Sanz B, Reisinger A, Zysset P. Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the randomized EuroGIOPs trial. J Bone Miner Res. 2013 doi: 10.1002/jbmr.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 32.Hofbauer LC, Rauner M. Minireview: live and let die: molecular effects of glucocorticoids on bone cells. Mol Endocrinol. 2009;23:1525–1531. doi: 10.1210/me.2009-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinstein RS. Glucocorticoids, osteocytes, and skeletal fragility: the role of bone vascularity. Bone. 2010;46:564–570. doi: 10.1016/j.bone.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ton FN, Gunawardene SC, Lee H, Neer RM. Effects of low-dose prednisone on bone metabolism. J Bone Miner Res. 2005;20:464–470. doi: 10.1359/JBMR.041125. [DOI] [PubMed] [Google Scholar]
- 35.Minisola S, Del Fiacco R, Piemonte S, Iorio M, Mascia ML, Fidanza F, Cipriani C, Raso I, Porfiri ML, Francucci CM, D'Erasmo E, Romagnoli E. Biochemical markers in glucocorticoid-induced osteoporosis. J Endocrinol Invest. 2008;31(7 Suppl):28–32. [PubMed] [Google Scholar]
- 36.Eastell R, Chen P, Saag KG, Burshell AL, Wong M, Warner MR, Krege JH. Bone formation markers in patients with glucocorticoid-induced osteoporosis treated with teriparatide or alendronate. Bone. 2010;46:929–934. doi: 10.1016/j.bone.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Graeff C, Marin F, Petto H, Kayser O, Reisinger A, Pena J, Zysset P, Gluer CC. High resolution quantitative computed tomography-based assessment of trabecular microstructure and strength estimates by finite-element analysis of the spine, but not DXA, reflects vertebral fracture status in men with glucocorticoid-induced osteoporosis. Bone. 2013;52:568–577. doi: 10.1016/j.bone.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 38.Graeff C, Timm W, Nickelsen TN, Farrerons J, Marín F, Barker C, Glüer CC; EUROFORS High Resolution Computed Tomography Substudy Group Monitoring teriparatide-associated changes in vertebral microstructure by high-resolution CT in vivo: results from the EUROFORS study. J Bone Miner Res. 2007;22:1426–1433. doi: 10.1359/jbmr.070603. [DOI] [PubMed] [Google Scholar]
- 39.Chevalier Y, Charlebois M, Pahra D, Varga P, Heini P, Schneider E, Zysset P. A patient-specific finite element methodology to predict damage accumulation in vertebral bodies under axial compression, sagittal flexion and combined loads. Comput Methods Biomech Biomed Engin. 2008;11:477–487. doi: 10.1080/10255840802078022. [DOI] [PubMed] [Google Scholar]
- 40.Keaveny TM. Biomechanical computed tomography—non-invasive bone strength analysis using clinical computed tomography scans. Ann N Y Acad Sci. 2010;1192:57–65. doi: 10.1111/j.1749-6632.2009.05348.x. [DOI] [PubMed] [Google Scholar]
- 41.Cosman F, Nieves JW, Zion M, Barbuto N, Lindsay R. Effect of prior and ongoing raloxifene therapy on response to PTH and maintenance of BMD after PTH therapy. Osteoporos Int. 2008;19:529–535. doi: 10.1007/s00198-007-0475-0. [DOI] [PubMed] [Google Scholar]
- 42.Recker RR, Marin F, Ish-Shalom S, Möricke R, Hawkins F, Kapetanos G, de la Peña MP, Kekow J, Farrerons J, Sanz B, Oertel H, Stepan J. Comparative effects of teriparatide and strontium ranelate on bone biopsies and biochemical markers of bone turnover in postmenopausal women with osteoporosis. J Bone Miner Res. 2009;24:1358–1368. doi: 10.1359/jbmr.090315. [DOI] [PubMed] [Google Scholar]
- 43.Stepan JJ, Burr DB, Li J, Ma YL, Petto H, Sipos A, Dobnig H, Fahrleitner-Pammer A, Michalska D, Pavo I. Histomorphometric changes by teriparatide in alendronate-pretreated women with osteoporosis. Osteoporos Int. 2010;21:2027–2036. doi: 10.1007/s00198-009-1168-7. [DOI] [PubMed] [Google Scholar]
- 44.Cosman F, Keaveny TM, Kopperdahl D, Wermers RA, Wan X, Krohn KD, Krege JH. Hip and spine strength effects of adding versus switching to teriparatide in postmenopausal women with osteoporosis treated with prior alendronate or raloxifene. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1853. [DOI] [PubMed] [Google Scholar]
- 45.Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051–1056. doi: 10.1359/jbmr.2003.18.6.1051. [DOI] [PubMed] [Google Scholar]
- 46.Bruyère O, Collette J, Rizzoli R, Decock C, Ortolani S, Cormier C, Detilleux J, Reginster JY. Relationship between 3-month changes in biochemical markers of bone remodelling and changes in bone mineral density and fracture incidence in patients treated with strontium ranelate for 3 years. Osteoporos Int. 2010;21:1031–1036. doi: 10.1007/s00198-009-1078-8. [DOI] [PubMed] [Google Scholar]
- 47.Melton LJ, 3rd, Riggs BL, Keaveny TM, Achenbach SJ, Hoffmann PF, Camp JJ, Rouleau PA, Bouxsein ML, Amin S, Atkinson EJ, Robb RA, Khosla S. Structural determinants of vertebral fracture risk. J Bone Miner Res. 2007;22:1885–1892. doi: 10.1359/jbmr.070728. [DOI] [PubMed] [Google Scholar]
- 48.Amin S, Kopperdhal DL, Melton LJ, Achenbach SJ, Therneua TM, Riggs BL, Keaveny TM. Khosla S (2011) Association of hip strength estimates by finite-element analysis with fractures in women and men. J Bone Miner Res. 2011;26:1593–1600. doi: 10.1002/jbmr.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keyak JH, Sigurdsson S, Karlsdottir G, Oskarsdottir D, Sigmarsdottir A, Zhao S, Kornak J, Harris TB, Sigurdsson G, Jonsson BY, Siggeirsdottir K, Eiriksdottir G, Gudnason V, Lang TF. Male–female differences in the association between incident hip fracture and proximal femoral strength: a finite element analysis study. Bone. 2011;48:1239–1245. doi: 10.1016/j.bone.2011.03.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheu Y, Zmuda JM, Boudreau RM, Petit MA, Ensrud KE, Bauer DC, Gordon CL, Orwoll ES, Cauley JA; Osteoporotic Fractures in Men MrOS Research Group Bone strength measured by peripheral quantitative computed tomography and the risk of nonvertebral fractures: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2011;26:63–71. doi: 10.1002/jbmr.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Sanyal A, Cawthon PM, Palermo L, Jekir M, Christensen J, Ensrud KE, Cummings SR, Orwoll E, Black DM; Osteoporotic Fractures in Men (MrOS) Research Group, Keaveny TM Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J Bone Miner Res. 2012;27:808–816. doi: 10.1002/jbmr.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vilayphiou N, Boutroy S, Szulc P, van Rietbergen B, Munoz F, Delmas PD, Chapurlat R. Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in men. J Bone Miner Res. 2011;26:965–973. doi: 10.1002/jbmr.297. [DOI] [PubMed] [Google Scholar]
- 53.Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000;85:3069–3076. doi: 10.1210/jc.85.9.3069. [DOI] [PubMed] [Google Scholar]
- 54.Orwoll ES, Scheele WH, Pual S, Adami S, Syversen U, Diez-Perez A, Kaufman J-M, Clancy AD, Gaich GA. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9–17. doi: 10.1359/jbmr.2003.18.1.9. [DOI] [PubMed] [Google Scholar]
- 55.Eastell R, Krege JH, Chen P, Glass EV, Reginster JY. Development of an algorithm for using PINP to monitor treatment of patients with teriparatide. Curr Med Res Opin. 2006;22:61–66. doi: 10.1185/030079905X75096. [DOI] [PubMed] [Google Scholar]
- 56.Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, Dalsky GP, Marcus R. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357:2028–2039. doi: 10.1056/NEJMoa071408. [DOI] [PubMed] [Google Scholar]
- 57.Glover SJ, Eastell R, McCloskey EV, Rogers A, Garnero P, Lowery J, Belleli R, Wright TM, John MR. Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone. 2009;45:1053–1058. doi: 10.1016/j.bone.2009.07.091. [DOI] [PubMed] [Google Scholar]