INTRODUCTION
Epilepsy is without question a costly and complicated major public health problem1. Also unquestioned is the fact that in addition to recurrent seizures, abnormalities in psychiatric status, cognition, and social-adaptive behaviors represent major sources of disability in children and adults with epilepsy–complications referred to collectively as the neurobehavioral comorbidities of the epilepsies. These comorbidities are not only problems in their own right, but also contribute to well-characterized difficulties in life performance (e.g., education, work, income)2. What has been controversial through the decades is the etiology of and best treatments for these comorbidities.
The goal here is to examine the neurobehavioral comorbidities and their potential mediators. Given that the incidence of epilepsy peaks in childhood and older adult years and the epilepsy is often chronic, coordinating care between pediatric and adult specialists is critical3. Therefore, we address the comorbidities in a lifespan perspective that is clinically useful and scientifically sound, identifying both strengths and limitations of the literature, and pointing to opportunities for intervention to improve the quality of life for both children and adults with epilepsy.
This review will proceed as follows (see Figure 1). First, we define the specific psychiatric, cognitive, and social comorbidities of pediatric and adult epilepsy, their epidemiology, and real life impact (Neurobehavioral Comorbidities). Second, we examine the relationship between epilepsy syndromes and the risk of neurobehavioral comorbidities (Epilepsy Syndromes). Third, we address the lifespan impact of epilepsy on brain neurodevelopment and brain aging and the risk of neurobehavioral comorbidities (Brain Development and Aging), followed by discussion of the overarching impact of broader brain disorders on both epilepsy and neurobehavioral comorbidities (Brain Disorders). Directions of causality are considered, as are the contribution of selected epilepsy-related characteristics (e.g., epileptiform discharges, age of onset, years of epilepsy chronicity, seizure medications). In the final section of the review, we outline clinic-friendly screening approaches for these problems and recommended pharmacological, behavioral, and educational interventions.
Figure 1.
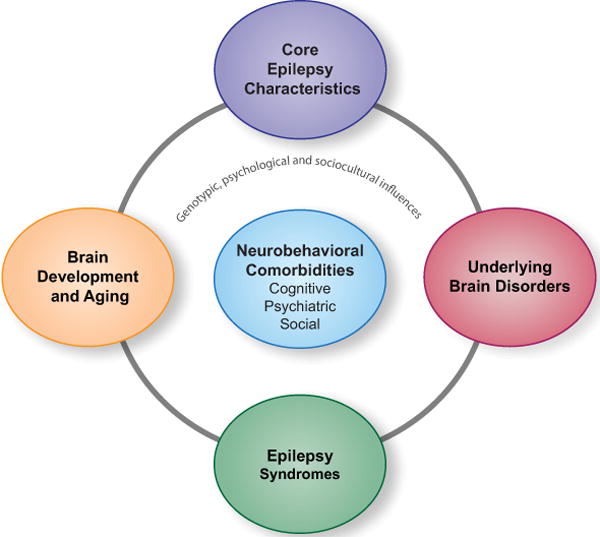
Theoretical framework for understanding the major mediators of the neurobehavioral comorbities of epilepsy (psychiatric, cognitive and social). The potential mediators include epilepsy syndrome, brain development and brain aging, underlying brain disorders and core epilepsy characteristics (e.g., early age of seizure onset, longer epilepsy duration, epileptiform discharges and seizure medication).
Increased risk of neurobehavioral comorbidities in epilepsy
The term comorbidity refers to a greater than coincidental presence of two conditions in the same person. Comorbidity does not infer a causal relationship, as co-occurrence of two disorders may arise by chance or share a common underlying mechanism. We focus here on population-and community-based studies, as these findings more representatively document the prevalence of psychiatric, cognitive, and social comorbidities of the epilepsies. This information is then supplemented with findings from specialized epilepsy centers, where typically more details can be provided regarding the nature, range, and correlates of these comorbidities.
Psychiatric comorbidities: Mood and anxiety disorders, psychotic disorder and attention deficit hyperactivity disorder
The burden of co-morbid psychiatric disorders is clear for both children and adults with epilepsy. Community- and population-based investigations uniformly report an increased prevalence of mood, anxiety, and other psychiatric disorders in epilepsy (Table 1).
TABLE 1.
Pediatric and Adult Psychiatric Epidemiological Studies
| Authors – Pediatrics | Primary Findings |
|---|---|
| Rutter et al. (1970)4 | CWE have higher rates of psychiatric disorder (55% complicated, 29% uncomplicated) vs. physical illnesses (12%) vs. HC (7%) |
| McDermott et al (1995)103 | CWE with elevated problems (31.4%) vs. cardiac problems (21.1%) and HC (8.5%) |
| Davies et al. (2003)5 | CWE with higher rates of psychiatric disorders (56% complicated epilepsy, 26.2% uncomplicated epilepsy) vs. diabetes (10.6%) and HC (9.3%) |
| Hoie et al. (2006)104 | CWE have 5–9 times more behavioral problems vs. HC |
| Lossius et al. (2006) 105 | CWE more behavior problems vs. HC (OR = 1.82) |
| Berg et al. (2007) 106 | CWE worse behavioral and competency scores vs. sibling controls |
| Alfstad et al. (2011)8 | CWE more likely to exhibit behavioral problems vs. HC (37.8% vs. 17%) |
| Russ et al. (2012) 10 | Increased depression, anxiety, ADHD, conduct, developmental delay, autistic spectrum disorder, social problems, parent aggravation |
| Cohen et al. (2012)107 | CWE have elevated rates of ADHD (27.2%) |
| Authors – Adults | |
| Ettinger (2004)108 | Depression more common in PWE (36.5%) compared to asthma (27.8%) and HC (11.8%). PWE had the most prior consultations and treatments for depression. |
| Ettinger (2005)109 | 48% of PWE screened positive for bipolar symptoms which was associated with significantly (p <.0001) more difficulties with work-related performance, social/leisure activities, and social/family interactions. |
| Tellez-Zentano et al. (2007) 110 | PWE more likely than individuals without epilepsy to report lifetime anxiety disorders or suicidal thoughts with odds ratio of 2.4 (95% CI = 1.5–3.8) and 2.2 (1.4–3.3), respectively. |
| Ottman et al. (2011) 111 | PWE more likely (p < 0.001) to report elevated anxiety, depression, bipolar disorder, attention-deficit/hyperactivity disorder, sleep disorder/apnea, and movement disorder/tremor (PR from 1.27–2.39); migraine headache, chronic pain, fibromyalgia, neuropathic pain (PR 1.36–1.96); and asthma (PR 1.25). |
| Kessler et al. (2011) 7 | PWE exhibit comorbid panic, PTSD, conduct disorder and substance use disorders. Comorbid disorders explain part of the association of epilepsy with impairment, but epilepsy remains associated with work disability, cognitive impairment and days of role impairment after controlling for comorbidities. |
| Rai et al (2012)6 | PWE exhibit elevated social phobia and agoraphobia, generalized anxiety disorder, depression, and measures of suicidality, these associations stronger than in asthma or diabetes, and similar to those in people with migraine or chronic headaches. |
Abbreviations: CWE, children with epilepsy; PWE, people with epilepsy; HC, healthy controls; CI, confidence interval; PR, prevalence ratio; ADHD, Attention Deficit Hyperactive Disorder; PTSD, Post-Traumatic Stress Disorder
In pediatrics, a unique set of unequivocal findings document the fact that psychiatric comorbidity is elevated in children with epilepsy compared to both the general population as well as children with other medical disorders. This elevated comorbidity is evident both in children with so-called uncomplicated epilepsies (normal neurological exam and intelligence, attending mainstream schools), but especially marked in those with complicated epilepsies (epilepsy plus brain lesion)—a set of findings reported in two independent epidemiological studies separated by 30-years.4,5
In adults, a recent United Kingdom population-based investigation involving 7,403 participants characterized the psychiatric burden associated with epilepsy6. After adjusting for confounders, people with epilepsy exhibited significantly elevated odds ratios for social phobia, agoraphobia, generalized anxiety disorder, and depression, as well as all measures of suicidality—these associations were significantly stronger than similar relationships in people with asthma or diabetes and comparable to chronic headache/migraine patients (Supplemental Figure 1).
As might be expected, psychiatric comorbidities in epilepsy are associated with more days of limitation and disability beyond that attributable to the epilepsy itself, and greater health care utilization and cost7. Given this psychiatric burden, it is unfortunate that routine screening is not a standard component of pediatric and adult care, especially in light of the potential lethality associated with depression and the widely documented increased risk of suicidal ideation, attempt, and completion6.
Cognitive comorbidities: Intelligence, academic achievement, and specific cognitive domains (e.g., executive function)
A significant potential complication of any human brain disorder, including epilepsy, is impairment in some aspect of objectively assessed cognition including intelligence, language, visuoperception, learning and memory, executive function, and/or processing speed.
Pediatric epilepsy benefits from several population and community-based investigations (Table 2). These studies indicate an increased prevalence of cognitive abnormalities in children with epilepsy, even those with uncomplicated epilepsies, compared to community- or population-based controls. This literature has also characterized prevalent academic achievement problems with significantly higher rates of school-based interventions (e.g., grade retention, summer school, tutors) and parent characterizations of the struggles of their children at school. We are not aware of a community- or population-based investigation of neuropsychological status in adults with epilepsy, clearly a major omission in this literature, but numerous clinical studies document this point8.
TABLE 2.
Pediatric Cognitive Epidemiological and Community Studies
| Authors | Primary Findings |
|---|---|
| Ellenberg et al. (1986)112 | IQ < 70 in 27% CWE |
| Hoie et al. (2005) 113 | Impaired nonverbal intelligence in 47% CWE vs. 3% HC |
| Hoie et al. (2006) 114 | Impaired executive function on 7 of 8 tests in CWE vs. HC |
| Berg et al. (2008) 115 | IQ < 80 in 25% of CWE |
| Fastenau et al. (2009) 116 | CWE worse on 4 of 4 cognitive domains vs. HC |
| Rantanen et al. (2010) 117 | Lower VIQ, FSIQ and learning in CWE (uncomplicated) vs. HC |
The burden of cognitive abnormality in the epilepsies is obviously significant. Investigations from clinical centers have addressed the details of the presenting cognitive profiles associated with specific epilepsy syndromes-a point to be reviewed later. Given this cognitive burden, cognitive screening should be a routine component of clinical care, a point advocated recently by an international review group9.
Social comorbidities: Adverse life performance outcomes
In addition to psychiatric and cognitive comorbidities, a germane issue is the negative impact of the epilepsies on real life performance, such as peer-to-peer interactions, marriage, independent living, employment and other facets of a productive life. The prevalence of social comorbidities in childhood onset epilepsies are reported in a nationally representative sample of 91,605 children in the United States (birth to 17 years) from the National Survey of Children’s Health, including 977 children reported by their parents to have been diagnosed with epilepsy. The children with epilepsy exhibited lower social competence (the ability to have productive and mutually satisfying relationships with others), more physical and functional disabilities, and more unmet medical and mental health needs10.
Similarly, real life burdens in adults with epilepsies are reported in community- and population-based studies. Especially important are findings from the 2005 Centers for Disease Control Behavioral Risk Factor Surveillance System11. Among 120,845 persons 18 years of age and older, there were 2,207 with a reported history of epilepsy (active in 919). Persons with epilepsy (and especially active epilepsy) exhibited significantly higher rates of unemployment, lower income (<$25K), less educational achievement, being single, and higher rates of problematic health conditions and lifestyle practices (e.g., obesity, inactivity, smoking). Persons with the most active epilepsy (i.e., seizures within the past 3 months) were most likely to report more mentally and physically unhealthy days and more activity limitations.
Multiple mediators of comorbidities
The prevalence of neurobehavioral comorbidities in epilepsy across the lifespan have triggered a concerted effort to uncover the potential mediators of these complications, which will inform treatment and prevention. Returning to Figure 1, we now examine the contribution of the type of epilepsy (Epilepsy Syndrome) on the risk of neurobehavioral comorbidities, a longstanding relationship of interest in the comorbidity literature. These studies highlight psychiatric, cognitive, and social impairments that are specific to the type of epilepsy, but also demonstrate some limitations of the syndrome classification approach. Next, we will delineate the impact of disrupted brain development, and accelerated brain aging, as well as broader considerations of intrinsic brain abnormalities on neurobehavioral comorbidities (Brain Development and Aging/Brain Disorder). Finally we will discuss psychosocial mediators of comorbidities.
The association between epilepsy syndromes and comorbidities
Therapeutic decisions and the prognosis of epilepsy rely on the accurate identification of specific epilepsy syndromes. Each syndrome consists of a constellation of clinical and laboratory variables (e.g. seizure semiology, age of onset, MRI, signature EEG findings, mode of inheritance) that set it apart from other disorders. Inherent in this assumption is that the core pathophysiology of each epilepsy syndrome governs both the type of manifested seizures and its associated neurobehavioral comorbidities. For decades, the syndromic model has served as the basis to investigate the cognitive and psychiatric domain(s) most at risk (Table 3).
TABLE 3.
Epilepsy Syndromes and Anticipated Cognitive and Psychiatric Complications
| Epilepsy Syndrome | Core Pathophysiology | Core Cognitive Deficit | Core Psychiatric Deficit |
|---|---|---|---|
| Temporal lobe epilepsy | Hippocampus and mesial temporal lobe | Anterograde memory | Depression and Anxiety |
| Frontal lobe epilepsy | Frontal lobe | Executive functions | Personality Disorders |
| Benign epilepsy with centrotemporal lobe | Sylvian and rolandic regions | Language abilities | Unknown |
| Absence epilepsy | Thalamocortical network | Attention | Unknown |
| Juvenile myoclonic epilepsy | Frontothalamic network | Executive function | Personality Disorders |
While it is logical to categorize comorbidities based on epilepsy syndromes, much remains to be learned about the distribution of shared versus syndrome-specific cognitive and psychiatric abnormalities given the lack of population-based investigations incorporating standardized, contemporary and comprehensive assessments. In point of fact, clinical evidence suggests that comorbidities do not necessarily respect pathophysiological boundaries. To exemplify this point, we first describe the spectrum of cognitive comorbidities that are unique to and common across selected epilepsy syndromes, followed by a discussion of associated psychiatric comorbidities. Then, we focus on temporal lobe epilepsy (TLE), the most frequent form of focal epilepsy, outlining a broad neuroanatomical basis for observed neurobehavioral comorbidities.
There has been a longstanding interest in the association between epilepsy syndromes and cognitive comorbidities. In Benign Epilepsy with Centrotemporal Spikes (BECTS), a syndrome with disturbances in the Sylvian and Rolandic regions, language problems are anticipated12, but a mild degree of deficit in attention and executive function are also noted13. In juvenile myoclonic epilepsy (JME), a disorder of frontothalamic hyperexcitability, impairments extend beyond attention and executive function to include verbal and visual memory, processing speed, naming and language function14. While the cognitive complications of childhood absence epilepsy (CAE) would be expected to predominantly involve attention given the underlying thalamocortical network derangement15, findings also demonstrate affected linguistic ability, broader executive function, and social competence16. While the cognitive complications of temporal lobe epilepsy (TLE) would be expected to center on memory disturbance given the underlying mesial temporal/hippocampal pathology, more widespread cognitive disorders have been reported including abnormal executive functions, processing speed, language, and other abilities17.
Similarly, the association between epilepsy syndromes and the risk of psychiatric comorbidities has been intensely studied, the best example being a proposed link between TLE and an increased risk for depression and psychosis. While depression and psychosis are known complications of TLE, these psychiatric comorbidities have been documented across a wide range of epilepsy syndromes, including BECTS18, JME19, and CAE20. Further, many of the epilepsy syndromes share broader psychiatric, behavioral, and social problems including Attention Deficit Hyperactive Disorder (ADHD), aggression, conduct problems, unemployment, and social isolation20, 21. This controversy regarding the unique psychiatric vulnerability of TLE, decades long, has been replaced by the view that psychiatric disorders are represented across diverse epilepsy syndromes.
These distributed psychiatric and cognitive abnormalities have been linked to surprisingly widespread neuroanatomical derangements. Such functional-structural relationships are again best characterized in people with TLE. While the link between the degree of hippocampal atrophy and memory deficits are well documented, considerable structural abnormality exists outside of this region, including extratemporal lobes (i.e. frontal, parietal and occipital lobes) as well as subcortical and cerebellar regions and their direct and indirect connections17. Importantly, these anatomical abnormalities are correlated with specific cognitive deficits, both within and beyond the memory domain. For example, decreased integrity of the uncinate fasciculus (a white matter tract between the mesial temporal lobe and the frontal lobe) is related to poorer memory performances22. Likewise, the volume of the corpus callosum and the integrity of the frontostriatal connections are correlated with executive function23, 24. Temporal lobe abnormalities have also been implicated in the neurocircuitry of depression and TLE. For example, the degree of hippocampal atrophy is inversely correlated with the severity and/or duration of depression in TLE with depression 25. Patients with TLE and psychosis had 16–18% larger amygdala volumes, when compared to TLE patients without psychosis26. In parallel with the cognitive literature, widespread functional and anatomical abnormalities are associated with mood disorders in TLE, including orbital frontal cortex27, cingulate gyrus28, 29, subcortical regions28, and brainstem29.
In summary, epilepsy syndromes provide a useful framework for considering the risk and type of comorbidities, but there is variability in comorbidity presentation within and across syndromes suggesting that variable phenotypic presentations are driven by other factors. At present, treating clinicians must be aware that pediatric and adult epilepsy syndromes may be associated with classically unanticipated cognitive, psychiatric, and social complications—this very true even for what have been viewed as uncomplicated epilepsies. A broad assessment of potential cognitive, psychiatric, and social complications is indicated to fully characterize associated comorbidities. It is certainly possible that as more clearly defined phenotypes are identified, more characteristic neurobehavioral profiles and targeted treatment strategies will result.
The impact of altered brain development and accelerated brain aging on comorbidities
That children with established and chronic epilepsies exhibit subtle anomalies in brain structure and connectivity has been shown. How these anomalies develop is an important question. In healthy maturing children, gray matter volumes decline with concomitant white matter volume expansion30 (Figure 2A). Against this dynamic backdrop, children with epilepsy exhibit abnormalities in brain structure at or near the time of seizure onset and an altered development trajectory early in the course of epilepsy. At baseline, quantitative MRI studies have revealed enlarged ventricular31 and reduced thalamic volumes32 in new-onset idiopathic generalized epilepsy. Over the prospective two years, children with epilepsy displayed a slowed white matter expansion and altered region-specific patterns of gray matter thinning, compared to age-matched controls33 (Figure 2B). Cross-sectional studies supplement this literature, demonstrating the adverse impact of early-onset epilepsy on brain structure. The posterior corpus callosum appears to be particularly vulnerable, with earlier age of seizure onset consistently linked to reduced white matter volume or microstructural integrity22, 23. Clearly, developmental trajectories in children are divergent from their healthy peers and evident near the time of epilepsy onset, but the link between aberrant brain development and variable cognitive, psychiatrc, and social progress in children with epilepsy is uncertain. Preliminary findings suggest that such a relationship exists. In children with new-onset JME, baseline frontothalamic volume is correlated with deficits in executive function34 and in children with new-onset BECTS, striatal enlargement predicted better executive function performances35. It remains uncertain to what degree an altered course of brain development is linked to reliable deviations in cognitive development, and whether these changes are permanent upon remission of seizures and cessation of treatment.
Figure 2.
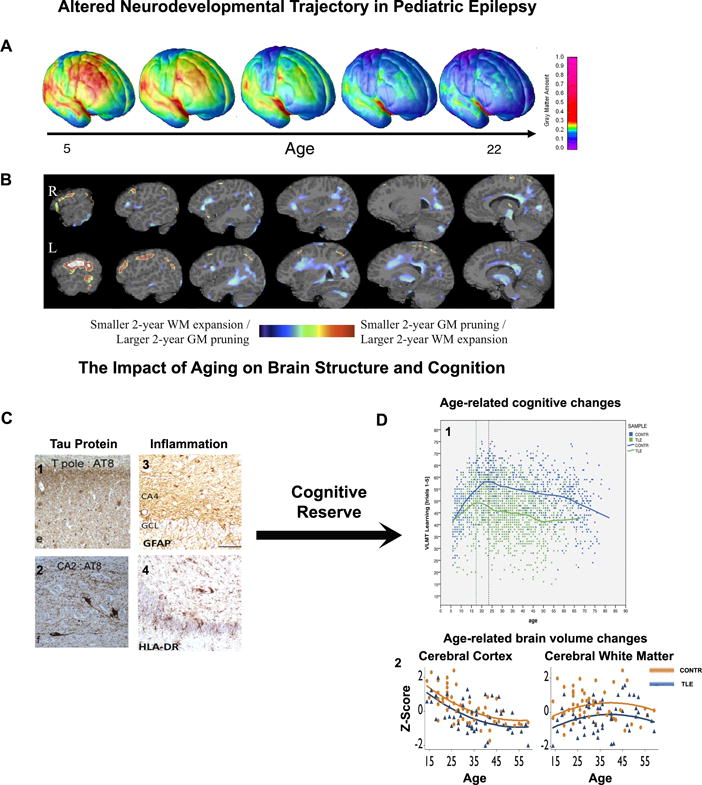
Altered neurodevelopmental trajectory in pediatric epilepsy. A) Gray matter pruning during normal brain development, with red areas denoting thicker and blue areas denoting thinner cortical regions30. B) Children with new-onset idiopathic generalized epilepsy over prospective 2 years exhibited reduced gray matter pruning (red regions) and white matter expansion (blue regions), when compared to their healthy peers33.
On the other end of the lifespan, the aging brain faces a different set of challenges with epilepsy36,. Aging-related brain atrophy reflects a myriad of cellular changes including a loss of dendritic spine, as well as accumulation of inflammatory damage37, and beta amyloid and tau protein38 (Figure 2C). These changes likely lead to increased vulnerability to seizure-induced cognitive deficits. Although potential cognitive decline in aging patients with epilepsy is likely very slow, these patients start at a lower baseline cognitive level than healthy subjects and may reach a clinically significant threshold of impairment earlier in life39 (Figure 2D). A particularly relevant question is whether cognitive reserve is associated with differential risks for cognitive impairment in elderly people. Greater cognitive reserve, as measured by factors such as higher intelligence, education attainment or greater occupational complexity, appears to improve or protect cognitive outcome in cross-sectional40 and 4-year prospective controlled studies41 (Figure 2 arrow). In summary, the accumulative effects of normal and pathological aging on brain health may influence the later cognitive outcome of aging persons with chronic epilepsy, but direct examination of these relationships are lacking.
The impact of underlying brain disorder on comorbidities
One of the fundamental assumptions in the field is that epilepsy does not occur in a normal brain. Indeed, factors relating to the underlying brain structural abnormalities not only increase the risk for epilepsy, but also for cognitive, psychiatric, and social impairments. Although the associated neurobehavioral comorbidities can be variable, the structural-functional relationships appears to be stronger in lesional epilepsy such as malformation of cortical development (MCD)42 and traumatic brain injury (TBI)43, as compared to epilepsy patients without structural abnormalities. For example, periventricular nodular heterotopia is an MCD associated with both epilepsy and dyslexia. Aberrant cortical to cortical white matter integrity has been found to be correlated with poor reading fluency42, while abnormal connections between the heterotopia and overlying cortex was related to longer seizures duration44. In TBI, the location and extent of injury are often closely related to the risk of developing epilepsy and cognitive complications. The presence of parietal lobe lesions and left insular involvement has been found to be associated with an increased risked of post-traumatic epilepsy and longer epilepsy duration was associated with a decline in full scale IQ43. In summary, the cause of epilepsy and the associated neuropathology often dictate the expression of cognitive comorbidities.
The link between an underlying brain disorder and psychiatric comorbidities has also emerged with evidence (Table 4) suggesting bidirectional relationships between epilepsy and a variety of cognitive, psychiatric, and social comorbidities in both pediatric and adult individuals. That is, psychiatric disorder may follow the onset of epilepsy, but, importantly, may also antedate incident epilepsy and serve as a “risk factor” for epilepsy- this is the case for depression, suicidality, ADHD, psychosis, and schizophrenia. Not only is there clear population-based evidence of shared susceptibilities between epilepsy and specific comorbidities, but family have revealed co-aggregation of cognitive45 and behavioral abnormalities46 in the siblings and/or parents of persons with epilepsy. Whereas these recent observations suggest that an intrinsic brain disorder, including a possible common genetic predisposition, may underlie both psychiatric comorbidities and epilepsy, the influence of environmental factors remains to be clarified. Furthermore it is unknown whether the co-aggregation of comorbidities is associated with similar structural or functional brain derangements, findings that have been demonstrated in other disorders such as siblings of patients with childhood onset schizophrenia47.
TABLE 4.
Bidirectional Psychiatric Disorder-Epilepsy Relationships
| Authors | |
|---|---|
| Fosgren (1990)118 | Depression a risk factor for epilepsy. |
| Hesdorffer et al. (2000)119 | Depression a risk factor for epilepsy. |
| Hesdorffer et al. (2004)120 | ADHD a risk factor for epilepsy in children. |
| Qin et al. (2005)121 | Association between epilepsy and schizophrenia. |
| Hesdorffer et al. (2006)122 | Depression and suicide attempt are risk factors for epilepsy. |
| McAfee et al. (2007)123 | Psychiatric disorders are risk factors for epilepsy in pediatrics. |
| Chang et al. (2011)124 | Bidirectional relationship between epilepsy and schizophrenia. |
| Adachi et al. (2011)125 | Bidirectional relationship between epilepsy and psychosis. |
| Jennum et al. (2011)126 | Higher health contacts, medical health care costs, lower work and antedate epilepsy onset. |
| Wotton & Goldacre (2012)127 | Bidirectional relationship between epilepsy and schizophrenia. |
| Adelow et al. (2012)128 | Bidirectional relationship of psychiatric hospitalization and epilepsy. |
| Hesdorffer et al. (In press)129 | Bidirectional relationship of psychosis, suicidality and epilepsy. |
One unstated but very clinically relevant implication of the bidirectional view is that neurobehavioral comorbidities may be present at diagnosis, early in the course of the disorder, and even occur prior to epilepsy onset. These effects have indeed been reported, especially in children, where multiple investigations of participants with epilepsy without clinical evidence of brain structural abnormalities have demonstrated a pattern of mild diffuse impairment across multiple cognitive domains, as well as elevated rates of behavioral problems including ADHD, with the suggestion that these problems antedated the first recognized seizure48, 49. Indeed, approximately 25% of children with new onset idiopathic epilepsies required special education services prior to clinical seizure onset50. In adults with new onset epilepsies, cognitive and behavioral disorders have also been reported51. From the clinical standpoint, the imperative would be to screen new onset cases for these issues in order to facilitate early intervention. That said, caveats regarding bidirectional hypotheses include the following: 1) similar bidirectional relationships have been reported for several chronic conditions including stroke, diabetes and cardiovascular disease, inferring a broad and not epilepsy-specific phenomenon52, 2) only a subset of persons with antecedent psychiatric disorders develop epilepsy and similarly a subset of those with new onset epilepsy present with or develop a psychiatric or cognitive comorbiditiy early on, so this relationship may apply to a unique phenotype.
The psychosocial impact of epilepsy
To this point we have focused largely on neurobiological biological mediators. However, the social and psychological impact of epilepsy can be profound. Narratives from people with epilepsy underscore the significant personal impact of exposure to essentially warningless events on the perception of personal control, the potential effect of epilepsy on many critical aspects of life (e.g., work and income, transportation, interpersonal relationships), exposure to real and perceived stigma, and the general fear of seizures53. These and other psychological, social and environmental factors influence the risk of psychiatric commorbities54 and need to be integrated into comprehensive predictive models.
Relationships between core epilepsy characteristics and comorbidities
Thus far, we have provided a brief account of the evidence that neurobehavioral comorbidities are influenced by several important factors including the epilepsy syndrome, the divergent effects of epilepsy on neurodevelopmental and aging trajectories, as well as the underlying brain disorder. Questions remain as to the directionality of these relationships. While the number of possible permutations are large, we will focus on selected links that are particularly relevant to clinical practice (Figure 1) – the contribution of core epilepsy characteristics, including epileptiform discharges55, the age of epilepsy onset56 and years of epilepsy chronicity57–59, and the prescribed medications, to neurobehavioral comorbidities.
Comorbidities are temporally influenced by epileptiform discharges and seizures
Interictal epileptiform discharges (IEDs) are abnormal neuronal discharges seen on the electroencephalogram (EEG) as a result of transient synchronous depolarization of neurons, producing trains of action potentials60. Whereas IEDs by definition occur when a patient is not having a seizure, they usually occur close to the seizure focus and are specific indicators of higher seizure risks61. The accumulative literature suggests that these discharges are not merely “harmless” biomarkers of epilepsy but have important behavioral and cognitive consequences. Cognitive deficits are related to the timing, duration, and spatial distribution of IEDs, with more pronounced impairment immediately before and after a prolonged (>3 seconds), generalized IED55. Although the most prominent effects of IEDs are attention and motor speed, transient cognitive impairment is not simply inattention55. Focal discharges have been temporally linked to material specific deficits, depending on the hemisphere (i.e. right-sided discharge associated with spatial memory deficits and left-side discharges related to verbal memory impairment55) and the particular brain region (occipital lobe discharges associated with decreased reaction time when material are presented in the visual field contralateral to the discharges62). However, applying these findings in the clinical setting is not straightforward. First, these cognitive deficits are ephemeral and long-term accumulative effects of IEDs are difficult to establish55. Second, pharmacological treatments to suppress IEDs, in addition to suppress seizures, have their own cognitive side effects (Table 5) and not all seizure medications can suppress IEDs. Finally, even when seizure medications suppress IEDs, corresponding cognitive improvements are not readily detected55, suggesting an effect of the underlying pathological substrate. Whereas the views differ regarding the targeted treatment of IEDs, all views agree that rendering individuals seizure free should be the primary objective, as seizure freedom is associated with reduced IEDs and improved cognition.
Table 5.
Cognitive and psychotrophic effects of seizure medications
| Medication | Motor/cognitive speed | Memory | Mood | Psychosis |
|---|---|---|---|---|
| Phenobarbital | − | − | − | ↔ |
|
| ||||
| Carbamazepine | − | − | +*/↔ | ↔ |
|
| ||||
| Phenytoin | − | − | −/↔ | − (Related to toxicity) |
|
| ||||
| Valporate | − | − | +*/↔ | ↔ |
|
| ||||
| Vigabatrin | ↔ | ↔ | − | − |
| Oxcarbazepine | ↔ | ↔ | +*/↔ | ↔ |
| Gabapentin | ↔ | ↔ | ↔ | |
| Lamotrigine | ↔ | ↔ | +*/↔ | |
| Levetriacetam | ↔ | ↔ | − | − |
| Pregabalin | −/ | ↔ | +*/↔ | ↔ |
| Topiramate | −/↔ | − | − | − |
| Tiagabine | − | − | − | − |
| Zonisamide | −/↔ | − | − | − |
−: negative effect; +: positive effect; ↔: no effect; 0: no evidence *data from psychiatry literature
In the case of psychoses, the temporal relationship with seizures is particularly relevant (Supplemental Figure 2). Peri-ictal psychoses are more frequently reported than inter-ictal psychoses. In particular, post-ictal psychoses account for approximately 25% of all psychoses of epilepsy. They usually occur after a cluster of secondary generalized tonic-clonic seizures in patients with TLE63. These psychotic episodes are usually brief, last from 1 to 6 days63, and often remit spontaneously within days or weeks. However, they are characterized by mixed mood and paranoid delusions with religious content, with an increased risk of self-harm64. Interictal psychoses are unrelated to seizures and typically develop after several years of active mesial TLE65. Despite their chronic course, differences with schizophrenia are quite striking with low rates of long-term institutionalization, a tendency of psychotic symptoms to attenuate over time, and the paucity of personality and cognitive deterioration66. Understanding these temporal relationships between seizures and psychosis are paramount, as specific types of epilepsy-related psychosis respond to different therapeutic strategies, a point we will elaborate in the treatment section. Finally, it is appreciated that there is an elevated time-limited risk of other psychiatric (e.g., depression) and cognitive (e.g., memory) complications in the post-ictal state that slowly resolve over time. The essential point is that the risk of neurobehavioral comorbidities may be transiently affected by intrinsic features of the disorder, a frequently overlooked consideration.
Early onset epilepsy is an important contributor of comorbidities
Although animal models suggest that seizures in the immature brain cause altered brain networks and poor cognition67, direct evidence of such an effect in humans has been elusive. When examining children with established epilepsy, many studies show earlier age of seizure onset to be correlated with poorer cognitive function. Specifically, early-life seizures are related to lower IQ68–70, poor academic performances71 and developmental delay72. However, firm conclusions regarding the impact of early-life seizures on cognition can be confounded by several factors: 1) Many studies are retrospective and cross-sectional in design, 2) Most studies included children with a wide spectrum of epilepsy syndromes including idiopathic and symptomatic as well as focal and generalized epilepsies68, 70, 3) Several investigations drew patients from specialized (e.g. surgical) populations with refractory epilepsy, which is difficult to apply to the general population of persons with epilepsy and uncomplicated epilepsy69, 73, 4) Earlier seizure onset can be highly correlated with longer seizure duration and many studies fail to disambiguate this co-linearity, 5) IQ is the only measure of cognitive outcome in many studies and the degree to which other cognitive domains are at risk remains to be determined.
In prospective studies tracking children from the onset of their epilepsy, some groups report subsets of children to be at risk of cognitive decline, but factors in addition to earlier age of seizure onset also appear to contribute to adverse cognitive outcomes74, 75 and there are mixed findings. Bourgeois and colleagues found that children with a decrement in IQ not only had earlier seizure onset but also had higher baseline IQ, higher incidence of seizure medication toxicity, and more refractory epilepsy74. In contrast, Oostrom and coworkers noted that persistent cognitive deficits were not associated with epilepsy-related factors but rather behavior problems prior to the diagnosis of epilepsy and a maladaptive family environment75. In summary, notwithstanding the small number of prospective investigations and their limitations, early-onset epilepsy appears to impart a greater risk for cognitive impairment than later-onset epilepsy, especially in children who have complicated epilepsy, behavior problems or difficult family environment.
Clinically, it is important to recognize that earlier seizure onset is an important but not sole contributor of neurobehavioral comobridities in children. Apparent from these studies is the need for a multidisciplinary team approach to aggressively control seizures, manage seizure medication toxicity, treat behavioral problems and mediate family environments.
Longer epilepsy chronicity is associated with lack of learning related improvements and development of psychiatric disorders
An important controversy is whether patients’ psychiatric status and cognitive abilities decline with increasing duration of epilepsy, a controversial literature that began in 1924 with mixed results59. The disparate findings have been influenced by small sample sizes, heterogeneous epilepsy syndromes, restricted and variable neuropsychological batteries (e.g., IQ and memory), and lack of control groups in many studies. Recent studies have aimed to address some of these shortcomings. One investigation highlighted the differential cognitive trajectories between TLE patients and healthy controls over a prospective 4-year interval41. Whereas control subjects showed broad test-retest improvements over time (practice effects), TLE patients on average exhibited minimal practice effects, suggesting a reduced capacity to learn from prior experience. Note too that within the TLE group, variability in cognitive outcomes existed with abnormal cognitive trajectories in a subset of patients (20–25%), with their abnormal prospective cognitive trajectories predicted by smaller baseline brain volumes, lower IQ, older chronological age, and longer epilepsy duration41.
Although the prospective controlled design is the optimal method to evaluate whether cognitive impairment is progressive, the study’s follow up time is short and the cognitive course over a life span is uncertain. Helmstaedter and Elger used a cross-sectional design to indirectly address this challenge, comparing the age-related regression of memory in 1156 TLE patients and 1000 healthy controls over a wide age span (6–80 years)39. The epilepsy group showed an earlier learning peak (defined as highest performance prior to decline) than the control group, but subsequent age-related declines ran parallel between the groups, with the epilepsy group always performing worse.
Progression is often couched in terms of cognitive decline. Less examined are the progressive psychiatric complications that may occur in the face of chronic epilepsy. Jones et al.76 examined temporal lobe epilepsy patients (n = 48) and healthy controls (n = 69) with structured psychiatric interview at baseline and 4 years later to characterize the course of DSM-IV Axis I disorders. Adjusting for the influence of prior psychiatric history, epilepsy subjects exhibited significantly greater risk of interval episodes of Total Axis I Disorders and Mood Disorders, indicating a poorer prognosis for these comorbidities in the context of chronic epilepsy.
While there may be divergent views regarding the long term psychiatric and cognitive course in chronic epilepsy, all views appear to agree on one important fact, which is that persons with chronic epilepsy appear to enter their elder years at a distinct cognitive disadvantage with what is best characterized as age-accelerated cognitive pathology, a point that was emphasized in the section regarding the impact of epilepsy on the aging brain.
Seizure medications have cognitive and psychotrophic effects
Given the large number of available seizure medications, selecting the appropriate medication becomes a daunting task for clinicians. Although there are clear guidelines as to which medication is efficacious for focal versus generalized epilepsy77, head to head comparisons within this broad classification of epilepsy are rare. In practice, selecting seizure medications depends as much on efficacy as on side effect profile. We, thus, summarized a general consensus on the cognitive and psychiatric side effects of seizure medications (Table 5)78. A few broad trends are noted. Cognitively, drug-induced impairments have been associated with barbiturates, phenytoin, and topiramate while milder cognitive effects are found for lamotrigine and oxcarbazepine. The link between depression and barbiturates, vigabatrin, and topiramate seems to be firmly established, as well as the mood stabilizing properties of carbamazepine, oxcarbazepine, lamotrigine, and valproate. In the majority of cases, a rapid titration of the drug, polypharmacy, a past history of a psychiatric disorder, and limbic system functional or structural abnormalities represent an increased risk for developing drug-induced cognitive and psychiatric complications.
Included in the psychiatric complications is the possible increased suicide risk associated with seizure medications, prompting the United States Food and Drug Administration to issue a warning79. Although this remains a controversial issue as studies on the topic have many confounders including a lack of consideration for past suicide attempts, what is clear is that a subgroup of people with epilepsy are more prone to develop psychiatric adverse event whenever a new seizure medication is introduced. Further, patients with a known history of depression and anxiety disorder experience more seizure medication related side effects than individuals without psychiatric diagnoses80. Therefore, screening for psychiatric comorbidities including suicidal thoughts and ideation with questionnaires such as the adverse event profile (AEP) should commence at the time of epilepsy diagnosis and prior to beginning treatment with seizure medications, with continual surveillance in company with medication titration and change.
CLINICAL AND TREATMENT CONSIDERATIONS
Typical and atypical features of mood, anxiety disorders, and psychosis
The phenomenology of psychiatric disorders associated with epilepsy has been a matter of debate with several implications for diagnosis, treatment and prognosis. Some authors contend that clinical syndromes comorbid to epilepsy are more often than not characterized by atypical features that are poorly reflected by conventional classificatory systems such as the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) and the International Classification of Disease (ICD-10)81. However, other studies demonstrate that it is possible to apply standardized criteria in a not negligible proportion of patients76, 82. In general terms, the psychopathological spectrum is likely to be large. On one hand, it is reasonable to hypothesize that patients with epilepsy can experience psychiatric disorders identical to those of patients without epilepsy. However, it is equally reasonable to assume that the underlying brain disorder might influence the expression of mood disorder symptoms, masking some features or emphasizing others. Concerning mood and anxiety disorders, we have reviewed in preceding sections a number of factors that may account for atypical features including: 1) the behavioral changes that may precede or follow seizures; 2) peculiar psychiatric syndromes such as the interictal dysphoric disorder; and 3) the psychotropic effect of seizure medications.
Screening and diagnosis of neurobehavioral comborbidities
To arrive at a definite diagnosis of a psychiatric syndrome in patients with epilepsy can be difficult. In fact, a number of symptoms, which are recognized as diagnostic criteria, may occur in epilepsy secondary to seizure activity or medication treatment. Moreover, one of the most frequent methodological errors in research studies on the subject is the sole reliance on clinical instruments that use measures or cut off scores that may not be valid in the epilepsy population. At present, very few measures exist that have been developed de novo for the assessment of comorbid psychopathology in epilepsy, using modern techniques of questionnaire development.
Among structured or semi-structured clinical interviews for diagnosis of psychiatric disorders following DSM criteria, Mintzer and Lopez 83 proposed the Epilepsy Addendum for Psychiatric Assessment (EAPA) to be used with the Mini International Neuropsychiatric Interview (MINI); a specific version of the Structured Clinical Interview for Axis I disorders (SCID-I) adapted for patients with epilepsy, named SCID-E, has been developed 84. Other clinical instruments have been developed specifically for patients with epilepsy in order to identify atypical manifestations of mood disorders not usually captured by DSM-based interviews such as the Seizure Questionnaire 85 and the Interictal Dysphoric Disorder Inventory 86. Among self-rating screening instruments for depressive symptoms, the well-known Beck Depression Inventory (BDI) has been validated in patients with epilepsy 87. A six-item, self-report questionnaire, the Neurological Disorders Depression Inventory for Epilepsy (NDDI-E), was developed for the rapid and objective detection of major depressive episodes in patients with epilepsy. It has been found to be a very practical and user-friendly screening instrument in outpatient epilepsy clinics 88.
Treatment of Psychiatric Disorders
Systematic data on treatment strategies for psychiatric disorders in epilepsy remain limited, with clinical practice relying heavily on individual experience. Experts from U.S. 89 and international90 panels advocate that treatment of primary psychiatric disorders outside epilepsy is valuable, if the underlying neurological condition is taken into consideration. In this regard, it is important to point out that it is still unclear whether patients with epilepsy respond equally to psychotropic medications or if they have different remission rates, compared to individuals without epilepsy.
Regarding mood disorders, the general impression is that depression in epilepsy is usually mild to moderate in severity with excellent response rates to adequate treatments. Selective serotonin reuptake inhibitors (SSRIs) (e.g. sertraline 50 mg or citalopram 20 mg) can be reasonably considered first choice, bearing in mind that drug doses need to be adjusted according to clinical response, especially if seizure medications with enzyme inducing properties are co-prescribed91. Fluvoxamine and nefazodone are the only difficult to use compounds because they are inhibitors of metabolic pathways of a number of seizure medications (especially carbamazepine, phenytoin)91.
Regarding psychoses, the treatment of peri-ictal psychoses is related to the treatment of the epilepsy (Figure 3). Neuroleptics can be used for a short period of time in order to reduce morbidity and mortality. Interictal psychoses may require long-lasting antipsychotic drug treatment. In such cases, patients need to be followed up in a psychiatric setting. Dosages should be always tailored to patient’s response because in almost all cases, enzyme inducers reduce the plasma levels of these drugs. In particular, the use of clozapine has to be carefully monitored because its metabolism has a high inter-individual and intra-individual variability and, especially in combination with valporate, interactions are difficult to predict92.
Figure 3.
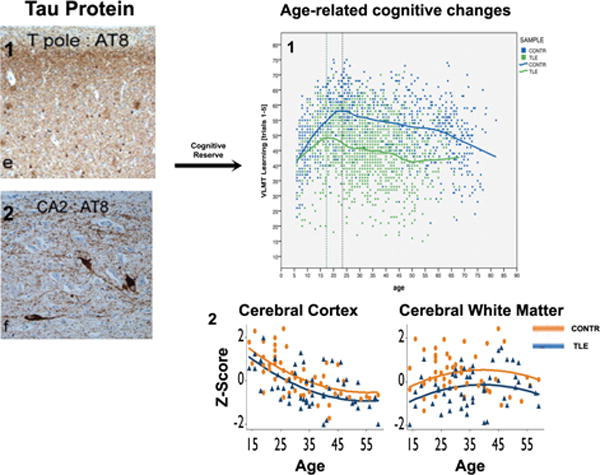
The impact of aging on brain structure and cognition. C) Accumulation of tau protein38 (stained with AT8) in the temporal pole (C1) hippocampus (C2) as well as inflammatory changes37 (stained with GFAP, C3 and HLA-DR, C4) in the hippocampus of patients with TLE. D) These age-related changes might increase epilepsy induced cognitive changes39 and brain atrophy130, in which patients start and persist at a lower cognitive level and brain volume than healthy controls. Cognitive reserve is a potential mediator of these age-related changes. Abbreviations: TLE, temporal lobe epilepsy; CONTR, controls; AT8, anti-phosphorylated tau antibody; GFAP, glial fibrillary acidic protein; HLA, antihuman leucocyte antigen. Part A modified with permission from National Academy of Science, USA; Parts B, C, D1 modified with permission from Oxford University Press; Part D2 modified with permission from Wiley-Blackwell.
The issue of worsening a patient’s seizures with initiation of psychotropic medications is a special concern for clinicians92. However, for the majority of compounds prescribed at dosages within the therapeutic range, the incidence of seizures is less than 0.5% when other risk factors are excluded. In fact, the “proconvulsive” effect is likely to be dose-dependent, becoming significant for very high dosages. Among antidepressants, SSRIs can be considered reasonably safe while clomipramine and maprotiline are the only drugs that may represent a concern. Among antipsychotics, chlorpromazine and clozapine are considered “proconvulsant” in epilepsy patients. The former may be a concern only at high doses (1000 mg/daily) and the latter at medium and high doses (>600 mg/daily). New compounds are usually well tolerated and can be considered reasonably safe. In particular, olanzapine and quetiapine showed a seizure rate of 0.9% and risperidone an even lower risk of seizures (about 0.3%). Finally, it should be acknowledged that the electroconvulsive therapy is not contraindicated in patients with epilepsy, it is well tolerated and worth considering in patients with very severe and treatment-resistant mood episodes.
Data on psychological therapies for mood and disorders in epilepsy appear promising. In general terms, cognitive behavioral therapy has showed utility in the management of depression in both adults93 and children94.
Treatment of Cognitive Disorders
In addition to psychiatric disorders, cognitive impairment also represents a major source of disability in people with epilepsy, but treatment options are more limited. One of the most practical approaches has been to minimize seizure medication side effects by utilizing a screening questionnaire such as the AEP. Rendering patients seizure free with a single agent at low dose would be the best strategy, as a recent study showed that their AEP was similar to controls95. In patients with refractory epilepsy, a tailored approach to select and adjust medications according to the patient’s individual AEP can reduce cognitive impairment and improve quality of life96.
Although medication adjustments can reduce cognitive deficits, they do not mitigate the underlying pathophysiology. Clearly, cognitive impairment in epilepsy has a distinctive mechanism from neurodengenerative diseases, as treatment with anticholinesterase inhibitor does not improve memory in people with epilepsy97. Recently, memory and cognitive training techniques have been applied to epilepsy and other neurological disorders, encompassing a wide range of strategies, including computer-assisted working memory programs, external aids (i.e. diaries, calendars), and exercises on self-regulation and problem solving activities. Helmstaedter and colleagues examined the impact of cognitive rehabilitation on 55 TLE patients after temporal lobe resection98. Positive effects of training were most evident in verbal learning, with little gain in long-term consolidation. Notably, performance improvements were only found in right TLE patients. These training improvements appears to be nonspecific as Koorenhof and coworkers found that 36% of TLE and 50% of control subjects had gains in memory test performances that were greater than expected from re-test or practice effect99. In summary, applying nonspecific strategies such as memory and attention trainings have produced mixed results and it is unclear why some people improve while others do not. Finally, it is also unclear if memory improvements has long-term benefits and would generalize to other cognitive domains.
Conclusions and future directions
Major advances have uncovered potential mediators of psychiatric, cognitive, and social comorbidities, but gaps remain in the early detection and treatment of these disorders. Whereas neurobehavioral comorbidities are present early in the course of epilepsy, standardized diagnostic and therapeutic modules are lacking to provide intervention. Brief, uniform, and clinic-friendly screening tools are needed to identify individuals at risk for neurobehavioral complications and homogenize their access to care. In this regard, the National Institute of Neurological Disorders and Stroke has provided leadership with funding of Neuro-QOL, a project aimed to develop psychometrically sound and clinical relevant quality of life measures in children and adult with neurological disorders100. Some of the instruments such questionnaires on executive function, depression and anxiety could be used to screen for comorbidities. However, the primary goal of these instruments is to facilitate longitudinal studies and large-scale clinical trials and thus the use in the clinical arena will require further validation.
Treatment options for neurobehavioral comorbidities remain to be developed. Aside from seizure medication management, strategies are largely adopted from the psychiatric literature and professional experiences. While similarities exit, distinctions are also present between psychiatric disorders and epilepsy-associated comorbidities. Further complicating the issue is that epilepsy is not a single disorder but encompasses a wide spectrum of complex conditions with shared and unique features. Thus, research is needed to clearly identify biomarkers for specific clinical phenotypes. Once the biological basis of different epilepsies is better understood, large-scale randomized trials are needed to develop epilepsy specific and individualized treatment options.
It is important to understand that epilepsy is a dynamic process in which damage likely invokes compensatory mechanisms. Whether we can harvest these altered neurocircuits to enhance cognitive and psychiatry rehabilitation is unknown, but animal models of TLE have provided considerable insight – early life seizures may lead to functional reorganization of the memory network in which successful performance of memory tasks requires increasing reliance on the frontal lobe, indicating a possible compensation for the dysfunctional medial temporal lobe101. Human imaging studies have revealed similar adaptive changes, demonstrating reduced functional connections within the temporal lobe and enhanced functional connections between the temporal and frontal lobes during working memory tasks in TLE102. Collectively, these studies hold the promise of a targeted intervention, leveraging epilepsy-specific compensatory mechanisms to enhance and accelerate psychiatric and cognitive recovery.
Supplementary Material
Supplemental Figure 1: Prevalence of psychiatric conditions in people with epilepsy6. Figure modified with permission from Wiley-Blackwell
Supplemental Figure 2: Clinical characteristics of psychoses of epilepsy. The figure depicts the frequency, EEG findings, level of consciousness, risk factors and treatment options for four types of psychosis in people with epilepsy.
Search Strategy.
PubMed was searched for English-language papers reporting cognitive, psychiatric or social complications of adult and child epilepsies.
Prior review papers of epilepsy and the cognitive, psychiatric and social comorbidities were examined.
Papers known by the authors to be pertinent to the topics at hand were reviewed.
Key Message Panel.
Cognitive, psychiatric and social problems are common comorbidities of childhood and adult epilepsies.
Epilepsy syndromes are relatively weak predictors of the cognitive, psychiatric and social complications of the epilepsies.
Seizure-related factors (e.g., EEG, age of seizure onset, epilepsy duration) provide additional prognostic value, but the underlying mediating mechanisms remain unclear,
Neurobehavioral comorbidities are evident at or prior to the onset of epilepsy in some patients, indicating the presence of antecedent factors that may include an abnormal neurological substrate.
Brain development and brain aging may be influenced by epilepsy, secondarily affecting the rate and type of associated neurobehavioral comorbidities.
Screening for cognitive, psychiatric and social comorbidities is critical in order to identify and facilitate treatment and improve quality of life.
Acknowledgments
Supported by grants from the National Institutes of Health including K23 NS060993 (JJL) and NINDS 2RO1-44351 (BPH). BPH also supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding source played no role in the interpretation of the studies present or the preparation and writing of this review.
Footnotes
Conflicts of Interest
MM has received consultancy fees from Pfizer, UCB Pharma and Janssen
JJL received a speaker’s honorarium from UCB-Pharma.
BPH has no potential conflicts to report.
References
- 1.Medicine Io, editor. Epilepsy across the spectrum. Washington, DC: The National Academy Press; 2012. [Google Scholar]
- 2.Chin RF, Cumberland PM, Pujar SS, Peckham C, Ross EM, Scott RC. Outcomes of childhood epilepsy at age 33 years: a population-based birth-cohort study. Epilepsia. 2011 Aug;52(8):1513–21. doi: 10.1111/j.1528-1167.2011.03170.x. [DOI] [PubMed] [Google Scholar]
- 3.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993 May-Jun;34(3):453–68. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 4.Rutter M, Graham P, Yule W. A neuropsychiatric study in childhood. London: S.I.M.P./William Heineman Medical Books; 1970. [Google Scholar]
- 5.Davies S, Heyman I, Goodman R. A population survey of mental health problems in children with epilepsy. Developmental medicine and child neurology. 2003 May;45(5):292–5. doi: 10.1017/s0012162203000550. [DOI] [PubMed] [Google Scholar]
- 6.Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia. 2012 Jun;53(6):1095–103. doi: 10.1111/j.1528-1167.2012.03500.x. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Lane MC, Shahly V, Stang PE. Accounting for comorbidity in assessing the burden of epilepsy among US adults: results from the National Comorbidity Survey Replication (NCS-R) Mol Psychiatry. 2011 May 17; doi: 10.1038/mp.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfstad KA, Clench-Aas J, Van Roy B, Mowinckel P, Gjerstad L, Lossius MI. Psychiatric symptoms in Norwegian children with epilepsy aged 8–13 years: effects of age and gender? Epilepsia. 2011 Jul;52(7):1231–8. doi: 10.1111/j.1528-1167.2011.03042.x. [DOI] [PubMed] [Google Scholar]
- 9.Parisi P, Moavero R, Verrotti A, Curatolo P. Attention deficit hyperactivity disorder in children with epilepsy. Brain Dev. 2010 Jan;32(1):10–6. doi: 10.1016/j.braindev.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics. 2012 Feb;129(2):256–64. doi: 10.1542/peds.2010-1371. [DOI] [PubMed] [Google Scholar]
- 11.Kobau R, Zahran H, Thurman DJ, Zack MM, Henry TR, Schachter SC, et al. Epilepsy surveillance among adults–19 States, behavioral risk factor surveillance system, 2005. MMWR Surveill Summ. 2008 Aug 8;57(6):1–20. [PubMed] [Google Scholar]
- 12.Monjauze C, Tuller L, Hommet C, Barthez MA, Khomsi A. Language in benign childhood epilepsy with centro-temporal spikes abbreviated form: rolandic epilepsy and language. Brain Lang. 2005 Mar;92(3):300–8. doi: 10.1016/j.bandl.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Kavros PM, Clarke T, Strug LJ, Halperin JM, Dorta NJ, Pal DK. Attention impairment in rolandic epilepsy: systematic review. Epilepsia. 2008 Sep;49(9):1570–80. doi: 10.1111/j.1528-1167.2008.01610.x. [DOI] [PubMed] [Google Scholar]
- 14.Pascalicchio TF, de Araujo Filho GM, da Silva Noffs MH, Lin K, Caboclo LO, Vidal-Dourado M, Ferreira Guilhoto LM, Yacubian EM. Neuropsychological profile of patients with juvenile myoclonic epilepsy: a controlled study of 50 patients. Epilepsy Behav. 2007 Mar;10(2):263–7. doi: 10.1016/j.yebeh.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002 Feb 15;22(4):1480–95. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, et al. Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities. Epilepsia. 2008 Nov;49(11):1838–46. doi: 10.1111/j.1528-1167.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 17.Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 2011 Mar;7(3):154–64. doi: 10.1038/nrneurol.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tovia E, Goldberg-Stern H, Ben Zeev B, Heyman E, Watemberg N, Fattal-Valevski A, et al. The prevalence of atypical presentations and comorbidities of benign childhood epilepsy with centrotemporal spikes. Epilepsia. 2011 Aug;52(8):1483–8. doi: 10.1111/j.1528-1167.2011.03136.x. [DOI] [PubMed] [Google Scholar]
- 19.Camfield CS, Camfield PR. Juvenile myoclonic epilepsy 25 years after seizure onset: a population-based study. Neurology. 2009 Sep 29;73(13):1041–5. doi: 10.1212/WNL.0b013e3181b9c86f. [DOI] [PubMed] [Google Scholar]
- 20.Caplan R, Siddarth P, Gurbani S, Hanson R, Sankar R, Shields WD. Depression and anxiety disorders in pediatric epilepsy. Epilepsia. 2005 May;46(5):720–30. doi: 10.1111/j.1528-1167.2005.43604.x. [DOI] [PubMed] [Google Scholar]
- 21.Hermann B, Jones J, Dabbs K, Allen CA, Sheth R, Fine J, et al. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain. 2007 Dec;130(Pt 12):3135–48. doi: 10.1093/brain/awm227. [DOI] [PubMed] [Google Scholar]
- 22.Riley JD, Franklin DL, Choi V, Kim RC, Binder DK, Cramer SC, et al. Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia. 2010 Apr;51(4):536–45. doi: 10.1111/j.1528-1167.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermann B, Hansen R, Seidenberg M, Magnotta V, O’Leary D. Neurodevelopmental vulnerability of the corpus callosum to childhood onset localization-related epilepsy. Neuroimage. 2003 Feb;18(2):284–92. doi: 10.1016/s1053-8119(02)00044-7. [DOI] [PubMed] [Google Scholar]
- 24.Riley JD, Moore S, Cramer SC, Lin JJ. Caudate atrophy and impaired frontostriatal connections are linked to executive dysfunction in temporal lobe epilepsy. Epilepsy Behav. 2011 May;21(1):80–7. doi: 10.1016/j.yebeh.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finegersh A, Avedissian C, Shamim S, Dustin I, Thompson PM, Theodore WH. Bilateral hippocampal atrophy in temporal lobe epilepsy: effect of depressive symptoms and febrile seizures. Epilepsia. 2011 Apr;52(4):689–97. doi: 10.1111/j.1528-1167.2010.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tebartz Van Elst L, Baeumer D, Lemieux L, Woermann FG, Koepp M, Krishnamoorthy S, et al. Amygdala pathology in psychosis of epilepsy: A magnetic resonance imaging study in patients with temporal lobe epilepsy. Brain. 2002 Jan;125(Pt 1):140–9. doi: 10.1093/brain/awf008. [DOI] [PubMed] [Google Scholar]
- 27.Butler T, Blackmon K, McDonald CR, Carlson C, Barr WB, Devinsky O, et al. Cortical thickness abnormalities associated with depressive symptoms in temporal lobe epilepsy. Epilepsy Behav. 2012 Jan;23(1):64–7. doi: 10.1016/j.yebeh.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Wu X, Lui S, Wu Q, Yao Z, Li Q, et al. Resting-state fMRI study of treatmentnaive temporal lobe epilepsy patients with depressive symptoms. Neuroimage. 2012 Mar;60(1):299–304. doi: 10.1016/j.neuroimage.2011.11.092. [DOI] [PubMed] [Google Scholar]
- 29.Lothe A, Didelot A, Hammers A, Costes N, Saoud M, Gilliam F, et al. Comorbidity between temporal lobe epilepsy and depression: a [18F]MPPF PET study. Brain. 2008 Oct;131(Pt 10):2765–82. doi: 10.1093/brain/awn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004 May 25;101(21):8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson DC, Irwin W, Dabbs K, Lin JJ, Jones JE, Hsu DA, et al. Ventricular enlargement in new-onset pediatric epilepsies. Epilepsia. 2011 Dec;52(12):2225–32. doi: 10.1111/j.1528-1167.2011.03323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pulsipher DT, Dabbs K, Tuchsherer V, Sheth RD, Koehn MA, Hermann BP, et al. Thalamofrontal neurodevelopment in new-onset pediatric idiopathic generalized epilepsy. Neurology. 2011 Jan 4;76(1):28–33. doi: 10.1212/WNL.0b013e318203e8f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tosun D, Dabbs K, Caplan R, Siddarth P, Toga A, Seidenberg M, et al. Deformation-based morphometry of prospective neurodevelopmental changes in new onset paediatric epilepsy. Brain. 2011 Apr;134(Pt 4):1003–14. doi: 10.1093/brain/awr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulsipher DT, Seidenberg M, Guidotti L, Tuchscherer VN, Morton J, Sheth RD, et al. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia. 2009 May;50(5):1210–9. doi: 10.1111/j.1528-1167.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin JJ, Riley JD, Hsu DA, Stafstrom CE, Dabbs K, Becker T, et al. Striatal hypertrophy and its cognitive effects in new-onset benign epilepsy with centrotemporal spikes. Epilepsia. 2012 Apr;53(4):677–85. doi: 10.1111/j.1528-1167.2012.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hermann B, Seidenberg M, Sager M, Carlsson C, Gidal B, Sheth R, et al. Growing old with epilepsy: the neglected issue of cognitive and brain health in aging and elder persons with chronic epilepsy. Epilepsia. 2008 May;49(5):731–40. doi: 10.1111/j.1528-1167.2007.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu JY, Thom M, Catarino CB, Martinian L, Figarella-Branger D, Bartolomei F, et al. Neuropathology of the blood-brain barrier and pharmaco-resistance in human epilepsy. Brain. 2012 Jun 28; doi: 10.1093/brain/aws147. [DOI] [PubMed] [Google Scholar]
- 38.Thom M, Liu JY, Thompson P, Phadke R, Narkiewicz M, Martinian L, et al. Neurofibrillary tangle pathology and Braak staging in chronic epilepsy in relation to traumatic brain injury and hippocampal sclerosis: a post-mortem study. Brain. 2011 Oct;134(Pt 10):2969–81. doi: 10.1093/brain/awr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helmstaedter C, Elger CE. Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain. 2009 Oct;132(Pt 10):2822–30. doi: 10.1093/brain/awp182. [DOI] [PubMed] [Google Scholar]
- 40.Jokeit H, Ebner A. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: a cross sectional study. J Neurol Neurosurg Psychiatry. 1999 Jul;67(1):44–50. doi: 10.1136/jnnp.67.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006 Jul;60(1):80–7. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- 42.Chang BS, Katzir T, Liu T, Corriveau K, Barzillai M, Apse KA, et al. A structural basis for reading fluency: white matter defects in a genetic brain malformation. Neurology. 2007 Dec 4;69(23):2146–54. doi: 10.1212/01.wnl.0000286365.41070.54. [DOI] [PubMed] [Google Scholar]
- 43.Raymont V, Salazar AM, Lipsky R, Goldman D, Tasick G, Grafman J. Correlates of posttraumatic epilepsy 35 years following combat brain injury. Neurology. 2010 Jul 20;75(3):224–9. doi: 10.1212/WNL.0b013e3181e8e6d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christodoulou JA, Walker LM, Del Tufo SN, Katzir T, Gabrieli JD, Whitfield-Gabrieli S, et al. Abnormal structural and functional brain connectivity in gray matter heterotopia. Epilepsia. 2012 Jun;53(6):1024–32. doi: 10.1111/j.1528-1167.2012.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith AB, Kavros PM, Clarke T, Dorta NJ, Tremont G, Pal DK. A neurocognitive endophenotype associated with rolandic epilepsy. Epilepsia. 2012 Jan 5; doi: 10.1111/j.1528-1167.2011.03371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hesdorffer DC, Caplan R, Berg AT. Familial clustering of epilepsy and behavioral disorders: evidence for a shared genetic basis. Epilepsia. 2012 Feb;53(2):301–7. doi: 10.1111/j.1528-1167.2011.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003 Apr;160(4):709–19. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- 48.Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics. 2001 Jan;107(1):115–22. doi: 10.1542/peds.107.1.115. [DOI] [PubMed] [Google Scholar]
- 49.Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”—a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003 Dec;112(6 Pt 1):1338–44. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- 50.Berg AT, Smith SN, Frobish D, Levy SR, Testa FM, Beckerman B, et al. Special education needs of children with newly diagnosed epilepsy. Developmental Medicine & Child Neurology. 2005 Nov;47(11):749–53. doi: 10.1017/S001216220500157X. [DOI] [PubMed] [Google Scholar]
- 51.Jacoby A, Lane S, Marson A, Baker GA. Relationship of clinical and quality of life trajectories following the onset of seizures: findings from the UK MESS Study. Epilepsia. 2011 May;52(5):965–74. doi: 10.1111/j.1528-1167.2010.02973.x. [DOI] [PubMed] [Google Scholar]
- 52.Baune BT, Stuart M, Gilmour A, Wersching H, Heindel W, Arolt V, et al. The relationship between subtypes of depression and cardiovascular disease: a systematic review of biological models. Transl Psychiatry. 2012;2:e92. doi: 10.1038/tp.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider JW, Conrad P. Having epilepsy: The experience and control of illness. Philadelphia: Temple University Press; 1983. [Google Scholar]
- 54.Hermann B, Jacoby A. The psychosocial impact of epilepsy in adults. Epilepsy Behav. 2009 Jun;15(Suppl 1):S11–6. doi: 10.1016/j.yebeh.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Binnie CD. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol. 2003 Dec;2(12):725–30. doi: 10.1016/s1474-4422(03)00584-2. [DOI] [PubMed] [Google Scholar]
- 56.Dikmen S, Matthews CG, Harley JP. The effect of early versus late onset of major motor epilepsy upon cognitive-intellectual performance. Epilepsia. 1975 Mar;16(1):73–81. doi: 10.1111/j.1528-1157.1975.tb04723.x. [DOI] [PubMed] [Google Scholar]
- 57.Jokeit H, Ebner A. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: a cross sectional study. J Neurol Neurosurg Psychiatry. 1999 Jul;67(1):44–50. doi: 10.1136/jnnp.67.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dodrill CB. Neuropsychological effects of seizures. Epilepsy Behav. 2004 Feb;5(Suppl 1):S21–4. doi: 10.1016/j.yebeh.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Seidenberg M, Pulsipher DT, Hermann B. Cognitive progression in epilepsy. Neuropsychol Rev. 2007 Dec;17(4):445–54. doi: 10.1007/s11065-007-9042-x. [DOI] [PubMed] [Google Scholar]
- 60.Prince DA, Connors BW. Mechanisms of interictal epileptogenesis. Adv Neurol. 1986;44:275–99. [PubMed] [Google Scholar]
- 61.de Curtis M, Librizzi L, Biella G. Discharge threshold is enhanced for several seconds after a single interictal spike in a model of focal epileptogenesis. Eur J Neurosci. 2001 Jul;14(1):174–8. doi: 10.1046/j.0953-816x.2001.01637.x. [DOI] [PubMed] [Google Scholar]
- 62.Shewmon DA, Erwin RJ. The effect of focal interictal spikes on perception and reaction time. II. Neuroanatomic specificity. Electroencephalogr Clin Neurophysiol. 1988 Apr;69(4):338–52. doi: 10.1016/0013-4694(88)90005-3. [DOI] [PubMed] [Google Scholar]
- 63.Logsdail SJ, Toone BK. Post-ictal psychoses. A clinical and phenomenological description. Br J Psychiatry. 1988 Feb;152:246–52. doi: 10.1192/bjp.152.2.246. [DOI] [PubMed] [Google Scholar]
- 64.Kanemoto K, Kawasaki J, Mori E. Violence and epilepsy: a close relation between violence and postictal psychosis. Epilepsia. 1999 Jan;40(1):107–9. doi: 10.1111/j.1528-1157.1999.tb01996.x. [DOI] [PubMed] [Google Scholar]
- 65.Adachi N, Matsuura M, Okubo Y, Oana Y, Takei N, Kato M, et al. Predictive variables of interictal psychosis in epilepsy. Neurology. 2000 Nov 14;55(9):1310–4. doi: 10.1212/wnl.55.9.1310. [DOI] [PubMed] [Google Scholar]
- 66.Fisekovic S, Burnazovic L. Epileptic psychoses – evaluation of clinical aspects. Bosn J Basic Med Sci. 2007 May;7(2):140–3. doi: 10.17305/bjbms.2007.3069. [DOI] [PubMed] [Google Scholar]
- 67.Bender RA, Baram TZ. Epileptogenesis in the developing brain: what can we learn from animal models? Epilepsia. 2007;48(Suppl 5):2–6. doi: 10.1111/j.1528-1167.2007.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bulteau C, Jambaque I, Viguier D, Kieffer V, Dellatolas G, Dulac O. Epileptic syndromes, cognitive assessment and school placement: a study of 251 children. Dev Med Child Neurol. 2000 May;42(5):319–27. doi: 10.1017/s0012162200000566. [DOI] [PubMed] [Google Scholar]
- 69.Vasconcellos E, Wyllie E, Sullivan S, Stanford L, Bulacio J, Kotagal P, et al. Mental retardation in pediatric candidates for epilepsy surgery: the role of early seizure onset. Epilepsia. 2001 Feb;42(2):268–74. doi: 10.1046/j.1528-1157.2001.12200.x. [DOI] [PubMed] [Google Scholar]
- 70.Rantanen K, Eriksson K, Nieminen P. Cognitive impairment in preschool children with epilepsy. Epilepsia. 2011 Aug;52(8):1499–505. doi: 10.1111/j.1528-1167.2011.03092.x. [DOI] [PubMed] [Google Scholar]
- 71.Schoenfeld J, Seidenberg M, Woodard A, Hecox K, Inglese C, Mack K, et al. Neuropsychological and behavioral status of children with complex partial seizures. Developmental medicine and child neurology. 1999 Nov;41(11):724–31. doi: 10.1017/s0012162299001486. [DOI] [PubMed] [Google Scholar]
- 72.Freitag H, Tuxhorn I. Cognitive function in preschool children after epilepsy surgery: rationale for early intervention. Epilepsia. 2005 Apr;46(4):561–7. doi: 10.1111/j.0013-9580.2005.03504.x. [DOI] [PubMed] [Google Scholar]
- 73.Smith ML, Elliott IM, Lach L. Cognitive, psychosocial, and family function one year after pediatric epilepsy surgery. Epilepsia. 2004 Jun;45(6):650–60. doi: 10.1111/j.0013-9580.2004.21903.x. [DOI] [PubMed] [Google Scholar]
- 74.Bourgeois BF, Prensky AL, Palkes HS, Talent BK, Busch SG. Intelligence in epilepsy: a prospective study in children. Ann Neurol. 1983 Oct;14(4):438–44. doi: 10.1002/ana.410140407. [DOI] [PubMed] [Google Scholar]
- 75.Oostrom KJ, van Teeseling H, Smeets-Schouten A, Peters AC, Jennekens-Schinkel A. Three to four years after diagnosis: cognition and behaviour in children with ‘epilepsy only’. A prospective, controlled study. Brain. 2005 Jul;128(Pt 7):1546–55. doi: 10.1093/brain/awh494. [DOI] [PubMed] [Google Scholar]
- 76.Jones JE, Bell B, Fine J, Rutecki P, Seidenberg M, Hermann B. A Controlled Prospective Investigation of Psychiatric Comorbidity in Temporal Lobe Epilepsy. Epilepsia. 2007 Jul 25;48(12):2357–60. doi: 10.1111/j.1528-1167.2007.01217.x. [DOI] [PubMed] [Google Scholar]
- 77.Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011 Sep 8;365(10):919–26. doi: 10.1056/NEJMra1004418. [DOI] [PubMed] [Google Scholar]
- 78.Mula M, Monaco F. Antiepileptic drugs and psychopathology of epilepsy: an update. Epileptic Disord. 2009 Mar;11(1):1–9. doi: 10.1684/epd.2009.0238. [DOI] [PubMed] [Google Scholar]
- 79.Mula M, Hesdorffer DC. Suicidal behavior and antiepileptic drugs in epilepsy: analysis of the emerging evidence. Drug Healthc Patient Saf. 2011;3:15–20. doi: 10.2147/DHPS.S13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kanner AM, Barry JJ, Gilliam F, Hermann B, Meador KJ. Depressive and anxiety disorders in epilepsy: do they differ in their potential to worsen common antiepileptic drug-related adverse events? Epilepsia. 2012 Jun;53(6):1104–8. doi: 10.1111/j.1528-1167.2012.03488.x. [DOI] [PubMed] [Google Scholar]
- 81.Krishnamoorthy ES, Trimble MR, Blumer D. The classification of neuropsychiatric disorders in epilepsy: a proposal by the ILAE Commission on Psychobiology of Epilepsy. Epilepsy Behav. 2007 May;10(3):349–53. doi: 10.1016/j.yebeh.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Jones JE, Hermann BP, Barry JJ, Gilliam F, Kanner AM, Meador KJ. Clinical assessment of Axis I psychiatric morbidity in chronic epilepsy: a multicenter investigation. J Neuropsychiatry Clin Neurosci. 2005 Spring;17(2):172–9. doi: 10.1176/jnp.17.2.172. [DOI] [PubMed] [Google Scholar]
- 83.Mintzer S, Lopez F. Comorbidity of ictal fear and panic disorder. Epilepsy Behav. 2002 Aug;3(4):330–7. doi: 10.1016/s1525-5050(02)00045-8. [DOI] [PubMed] [Google Scholar]
- 84.Krishnamoorthy ES. The evaluation of behavioral disturbances in epilepsy. Epilepsia. 2006;47(Suppl 2):3–8. doi: 10.1111/j.1528-1167.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 85.Blumer D, Montouris G, Davies K, Wyler A, Phillips B, Hermann B. Suicide in epilepsy: psychopathology, pathogenesis, and prevention. Epilepsy Behav. 2002 Jun;3(3):232–41. doi: 10.1016/s1525-5050(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 86.Mula M, Jauch R, Cavanna A, Collimedaglia L, Barbagli D, Gaus V, et al. Clinical and psychopathological definition of the interictal dysphoric disorder of epilepsy. Epilepsia. 2008 Apr;49(4):650–6. doi: 10.1111/j.1528-1167.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- 87.Jones JE, Hermann BP, Woodard JL, Barry JJ, Gilliam F, Kanner AM, et al. Screening for major depression in epilepsy with common self-report depression inventories. Epilepsia. 2005 May;46(5):731–5. doi: 10.1111/j.1528-1167.2005.49704.x. [DOI] [PubMed] [Google Scholar]
- 88.Gilliam FG, Barry JJ, Hermann BP, Meador KJ, Vahle V, Kanner AM. Rapid detection of major depression in epilepsy: a multicentre study. Lancet Neurol. 2006 May;5(5):399–405. doi: 10.1016/S1474-4422(06)70415-X. [DOI] [PubMed] [Google Scholar]
- 89.Barry JJ, Ettinger AB, Friel P, Gilliam FG, Harden CL, Hermann B, et al. Consensus statement: the evaluation and treatment of people with epilepsy and affective disorders. Epilepsy Behav. 2008 Jul;13(Suppl 1):S1–29. doi: 10.1016/j.yebeh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 90.Kerr MP, Mensah S, Besag F, de Toffol B, Ettinger A, Kanemoto K, et al. International consensus clinical practice statements for the treatment of neuropsychiatric conditions associated with epilepsy. Epilepsia. 2011 Nov;52(11):2133–8. doi: 10.1111/j.1528-1167.2011.03276.x. [DOI] [PubMed] [Google Scholar]
- 91.Mula M. Anticonvulsants – antidepressants pharmacokinetic drug interactions: the role of the CYP450 system in psychopharmacology. Curr Drug Metab. 2008 Oct;9(8):730–7. doi: 10.2174/138920008786049311. [DOI] [PubMed] [Google Scholar]
- 92.Mula M, Monaco F. Antiepileptic-antipsychotic drug interactions: a critical review of the evidence. Clin Neuropharmacol. 2002 Sep-Oct;25(5):280–9. doi: 10.1097/00002826-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 93.Crail-Melendez D, Herrera-Melo A, Martinez-Juarez IE, Ramirez-Bermudez J. Cognitive-behavioral therapy for depression in patients with temporal lobe epilepsy: a pilot study. Epilepsy Behav. 2012 Jan;23(1):52–6. doi: 10.1016/j.yebeh.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 94.Martinovic Z. Adjunctive behavioural treatment in adolescents and young adults with juvenile myoclonic epilepsy. Seizure. 2001 Jan;10(1):42–7. doi: 10.1053/seiz.2000.0479. [DOI] [PubMed] [Google Scholar]
- 95.Perucca P, Jacoby A, Marson AG, Baker GA, Lane S, Benn EK, et al. Adverse antiepileptic drug effects in new-onset seizures: a case-control study. Neurology. 2011 Jan 18;76(3):273–9. doi: 10.1212/WNL.0b013e318207b073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gilliam FG, Fessler AJ, Baker G, Vahle V, Carter J, Attarian H. Systematic screening allows reduction of adverse antiepileptic drug effects: a randomized trial. Neurology. 2004 Jan 13;62(1):23–7. doi: 10.1212/wnl.62.1.23. [DOI] [PubMed] [Google Scholar]
- 97.Hamberger MJ, Palmese CA, Scarmeas N, Weintraub D, Choi H, Hirsch LJ. A randomized, double-blind, placebo-controlled trial of donepezil to improve memory in epilepsy. Epilepsia. 2007 Jul;48(7):1283–91. doi: 10.1111/j.1528-1167.2007.01114.x. [DOI] [PubMed] [Google Scholar]
- 98.Helmstaedter C, Loer B, Wohlfahrt R, Hammen A, Saar J, Steinhoff BJ, et al. The effects of cognitive rehabilitation on memory outcome after temporal lobe epilepsy surgery. Epilepsy Behav. 2008 Apr;12(3):402–9. doi: 10.1016/j.yebeh.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 99.Koorenhof L, Baxendale S, Smith N, Thompson P. Memory rehabilitation and brain training for surgical temporal lobe epilepsy patients: a preliminary report. Seizure. 2012 Apr;21(3):178–82. doi: 10.1016/j.seizure.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 100.Cella D, Lai JS, Nowinski CJ, Victorson D, Peterman A, Miller D, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012 Jun 5;78(23):1860–7. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kleen JK, Wu EX, Holmes GL, Scott RC, Lenck-Santini PP. Enhanced oscillatory activity in the hippocampal-prefrontal network is related to short-term memory function after early-life seizures. J Neurosci. 2011 Oct 26;31(43):15397–406. doi: 10.1523/JNEUROSCI.2196-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Campo P, Garrido MI, Moran RJ, Maestu F, Garcia-Morales I, Gil-Nagel A, et al. Remote Effects of Hippocampal Sclerosis on Effective Connectivity during Working Memory Encoding: A Case of Connectional Diaschisis? Cereb Cortex. 2011 Aug 1; doi: 10.1093/cercor/bhr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McDermott S, Mani S, Krishnaswami S. A population-based analysis of specific behavior problems associated with childhood seizures. Journal of Epilepsy. 1995;8(2):110. [Google Scholar]
- 104.Hoie B, Sommerfelt K, Waaler PE, Alsaker FD, Skeidsvoll H, Mykletun A. Psychosocial problems and seizure-related factors in children with epilepsy. Developmental medicine and child neurology. 2006 Mar;48(3):213–9. doi: 10.1017/S0012162206000454. [DOI] [PubMed] [Google Scholar]
- 105.Lossius MI, Clench-Aas J, van Roy B, Mowinckel P, Gjerstad L. Psychiatric symptoms in adolescents with epilepsy in junior high school in Norway: a population survey. Epilepsy Behav. 2006 Sep;9(2):286–92. doi: 10.1016/j.yebeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 106.Berg AT, Vickrey BG, Testa FM, Levy SR, Shinnar S, DiMario F. Behavior and social competency in idiopathic and cryptogenic childhood epilepsy. Dev Med Child Neurol. 2007 Jul;49(7):487–92. doi: 10.1111/j.1469-8749.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 107.Cohen R, Senecky Y, Shuper A, Inbar D, Chodick G, Shalev V, et al. Prevalence of Epilepsy and Attention-Deficit Hyperactivity (ADHD) Disorder: A Population-Based Study. J Child Neurol. 2012 May 1; doi: 10.1177/0883073812440327. [DOI] [PubMed] [Google Scholar]
- 108.Ettinger A, Reed M, Cramer J. Depression and comorbidity in community-based patients with epilepsy or asthma. Neurology. 2004 Sep 28;63(6):1008–14. doi: 10.1212/01.wnl.0000138430.11829.61. [DOI] [PubMed] [Google Scholar]
- 109.Ettinger AB, Reed ML, Goldberg JF, Hirschfeld RM. Prevalence of bipolar symptoms in epilepsy vs other chronic health disorders. Neurology. 2005 Aug 23;65(4):535–40. doi: 10.1212/01.wnl.0000172917.70752.05. [DOI] [PubMed] [Google Scholar]
- 110.Tellez-Zenteno JF, Patten SB, Jette N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007 Dec;48(12):2336–44. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 111.Ottman R, Lipton RB, Ettinger AB, Cramer JA, Reed ML, Morrison A, et al. Comorbidities of epilepsy: results from the Epilepsy Comorbidities and Health (EPIC) survey. Epilepsia. 2011 Feb;52(2):308–15. doi: 10.1111/j.1528-1167.2010.02927.x. [DOI] [PubMed] [Google Scholar]
- 112.Ellenberg JH, Hirtz DG, Nelson KB. Do seizures in children cause intellectual deterioration? N Engl J Med. 1986 Apr 24;314(17):1085–8. doi: 10.1056/NEJM198604243141705. [DOI] [PubMed] [Google Scholar]
- 113.Hoie B, Mykletun A, Sommerfelt K, Bjornaes H, Skeidsvoll H, Waaler PE. Seizure-related factors and non-verbal intelligence in children with epilepsy. A population-based study from Western Norway. Seizure. 2005 Jun;14(4):223–31. doi: 10.1016/j.seizure.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 114.Hoie B, Mykletun A, Waaler PE, Skeidsvoll H, Sommerfelt K. Executive functions and seizure-related factors in children with epilepsy in Western Norway. Dev Med Child Neurol. 2006 Jun;48(6):519–25. doi: 10.1017/S0012162206001095. [DOI] [PubMed] [Google Scholar]
- 115.Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, et al. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008 Apr;49(4):608–14. doi: 10.1111/j.1528-1167.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 116.Fastenau PS, Johnson CS, Perkins SM, Byars AW, deGrauw TJ, Austin JK, et al. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology. 2009 Aug 18;73(7):526–34. doi: 10.1212/WNL.0b013e3181b23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rantanen K, Nieminen P, Eriksson K. Neurocognitive functioning of preschool children with uncomplicated epilepsy. J Neuropsychol. 2010 Mar;4(Pt 1):71–87. doi: 10.1348/174866409X451465. [DOI] [PubMed] [Google Scholar]
- 118.Forsgren L, Nystrom L. An incident case-referent study of epileptic seizures in adults. Epilepsy Res. 1990 May-Jun;6(1):66–81. doi: 10.1016/0920-1211(90)90010-s. [DOI] [PubMed] [Google Scholar]
- 119.Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Ann Neurol. 2000 Feb;47(2):246–9. [PubMed] [Google Scholar]
- 120.Hesdorffer DC, Ludvigsson P, Olafsson E, Gudmundsson G, Kjartansson O, Hauser WA. ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Arch Gen Psychiatry. 2004 Jul;61(7):731–6. doi: 10.1001/archpsyc.61.7.731. [DOI] [PubMed] [Google Scholar]
- 121.Qin P, Xu H, Laursen TM, Vestergaard M, Mortensen PB. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. Bmj. 2005 Jul 2;331(7507):23. doi: 10.1136/bmj.38488.462037.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Annals of Neurology. 2006 Jan;59(1):35–41. doi: 10.1002/ana.20685. [DOI] [PubMed] [Google Scholar]
- 123.McAfee AT, Chilcott KE, Johannes CB, Hornbuckle K, Hauser WA, Walker AM. The incidence of first provoked and unprovoked seizure in pediatric patients with and without psychiatric diagnoses. Epilepsia. 2007 Jun;48(6):1075–82. doi: 10.1111/j.1528-1167.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 124.Chang YT, Chen PC, Tsai IJ, Sung FC, Chin ZN, Kuo HT, et al. Bidirectional relation between schizophrenia and epilepsy: a population-based retrospective cohort study. Epilepsia. 2011 Nov;52(11):2036–42. doi: 10.1111/j.1528-1167.2011.03268.x. [DOI] [PubMed] [Google Scholar]
- 125.Adachi N, Onuma T, Kato M, Ito M, Akanuma N, Hara T, et al. Analogy between psychosis antedating epilepsy and epilepsy antedating psychosis. Epilepsia. 2011 Jul;52(7):1239–44. doi: 10.1111/j.1528-1167.2011.03039.x. [DOI] [PubMed] [Google Scholar]
- 126.Jennum P, Gyllenborg J, Kjellberg J. The social and economic consequences of epilepsy: a controlled national study. Epilepsia. 2011 May;52(5):949–56. doi: 10.1111/j.1528-1167.2010.02946.x. [DOI] [PubMed] [Google Scholar]
- 127.Wotton CJ, Goldacre MJ. Coexistence of schizophrenia and epilepsy: Record-linkage studies. Epilepsia. 2012 Apr;53(4):e71–4. doi: 10.1111/j.1528-1167.2011.03390.x. [DOI] [PubMed] [Google Scholar]
- 128.Adelow C, Andersson T, Ahlbom A, Tomson T. Hospitalization for psychiatric disorders before and after onset of unprovoked seizures/epilepsy. Neurology. 2012 Feb 7;78(6):396–401. doi: 10.1212/WNL.0b013e318245f461. [DOI] [PubMed] [Google Scholar]
- 129.Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA. Epilepsy, suicidality, and psychiatric disorders: A bidirectional association. Annals of Neurology. doi: 10.1002/ana.23601. In Press. [DOI] [PubMed] [Google Scholar]
- 130.Dabbs K, Becker T, Jones J, Rutecki P, Seidenberg M, Hermann B. Brain structure and aging in chronic temporal lobe epilepsy. Epilepsia. 2012;53:1033–43. doi: 10.1111/j.1528-1167.2012.03447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Prevalence of psychiatric conditions in people with epilepsy6. Figure modified with permission from Wiley-Blackwell
Supplemental Figure 2: Clinical characteristics of psychoses of epilepsy. The figure depicts the frequency, EEG findings, level of consciousness, risk factors and treatment options for four types of psychosis in people with epilepsy.


