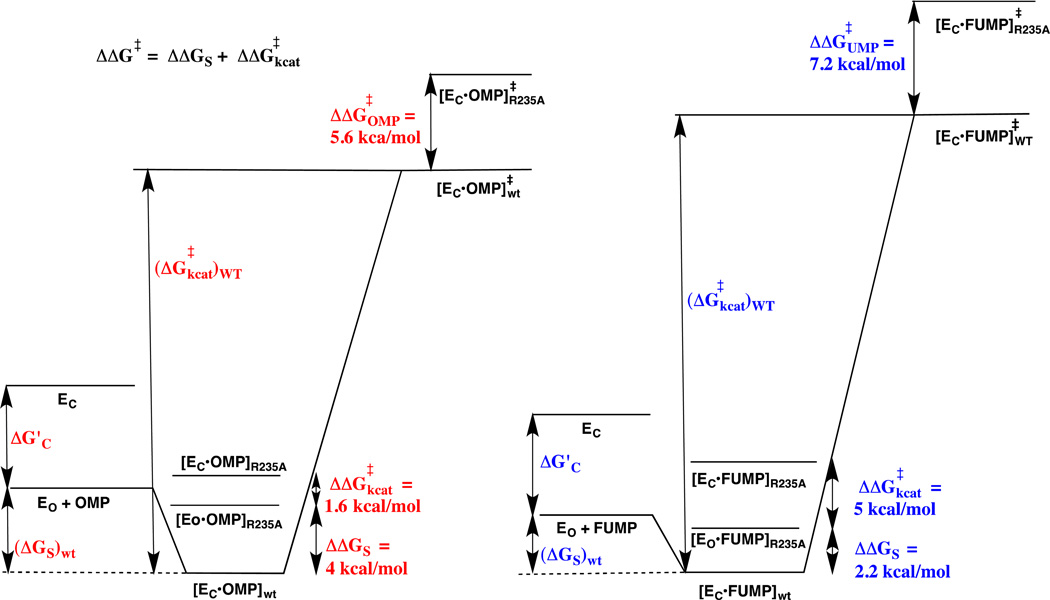
Figure 8.
Free energy reaction profiles that illustrate the different effects of the R235A mutation on the kinetic parameters for ScOMPDC-catalyzed decarboxylation of OMP to form UMP (profile on left) and the deuterium exchange reaction of h-FUMP to form d-FUMP (profile on right). The binding energy of R235A and other residues in the phosphodianion gripper loop is utilized to drive the conversion of open enzyme EO to closed enzyme EC – this binding energy is partly expressed at the Michaelis complex when loop closure at the Michaelis complex is thermodynamically favorable (Kc > 1, eq 4 – 6). The substrate binding energy (ΔGS)wt of OMP is greater than for FUMP, and we propose that this reflects the utilization of the binding interactions with the -CO2− group of OMP to drive the conversion of EO to EC (ΔG’c). The overall effect of the R235A mutation on the barrier for the deuterium exchange (ΔΔG‡UMP = 7.2 kcal/mol) is larger than for the decarboxylation reaction (ΔΔG‡OMP = 5.6 kcal/mol). The R235A mutation results mainly in a decrease in ΔGS for the decarboxylation reaction (ΔΔGS = 4 kcal/mole). We propose that the Michaelis complex between OMP and the R235A mutant enzyme exists mainly in the nonproductive open form ([Eo•OMP]R235A), because there is insufficient binding energy at this Michaelis complex enzyme to drive the enzyme conformational change (Kc < 1, eq 4–6). The effect of the R235A mutation on kcat (ΔΔG‡kcat = 1.6 kcal/mol) is shown as the 1.6 kcal/mol barrier for conversion of [Eo•OMP]R235A to the productive complex ([EC•OMP]R235A). The smaller binding energy from FUMP compared with OMP available to drive the enzyme conformational change ((Kc)FUMP < (Kc)OMP, eq 4 – 6) results in the smaller the effect of the R235A mutation on ΔGS (ΔΔGS = 2.2 kcal/mol) and a large 5 kcal/mol barrier to conversion of nonproductive [Eo•FUMP]R235A to productive ([EC•FUMP]R235A that is added to the barrier for conversion of [Eo•FUMP]R235A to product (ΔΔG‡kcat = 5 kcal/mol).