Figure 4.
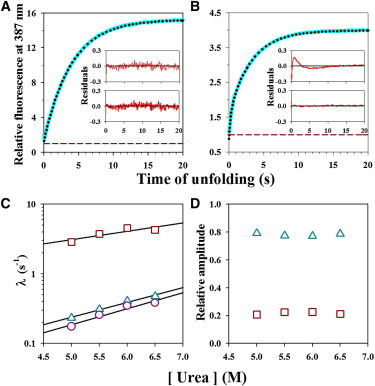
Two-site FRET monitored unfolding kinetics in 5.5 M urea and 10°C. (A and B) Representative kinetic unfolding traces (cyan lines) monitored by measurement of fluorescence intensity at 387 nm for Cys-82-TNB (panel A) and Cys-25-TNB (panel B). In each panel, the dotted black line through the data represents a fit to a single and two-exponential equation for Cys-82-TNB and Cys-25-TNB, respectively. The data are normalized to the native signal of 1 for each labeled protein. In each panel, the upper inset and the lower inset show the residuals of fitting of the kinetic traces to single and two-exponential equations, respectively. (C and D) Dependence on urea concentration of the FRET-monitored kinetics of unfolding. The rate constants of unfolding for Cys-82-TNB (o), and for the slow (Δ) and fast (□) phases of unfolding for Cys-25-TNB are shown at different urea concentrations (panel C). The black lines through the data represent fits to a linear equation. Relative amplitudes of the slow (Δ) and fast (□) phases of unfolding for Cys-25-TNB are shown as a function of urea concentration (panel D). To see this figure in color, go online.
