Figure 2.
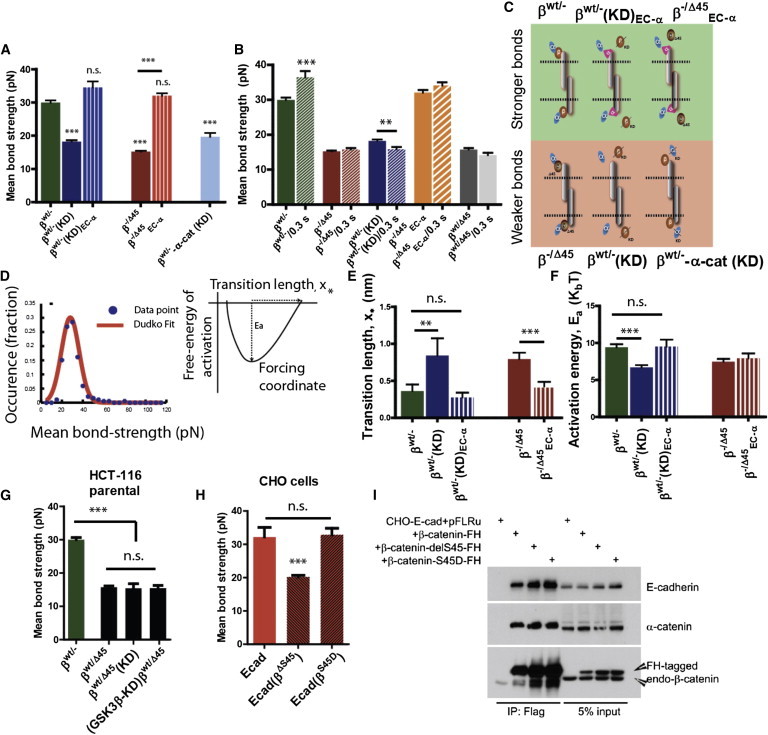
β-catenin modulates the strength of single intercellular Ecad/Ecad bonds. (A) Strength of Ecad/Ecad bonds is significantly weakened in HCT116 cells expressing only a mutant β-catenin with S45 deleted (β−/Δ45, plain red) and in HCT116 cells wherein β-catenin is knocked down (βWT/−(KD), plain blue) as compared to cells expressing only WT β-catenin (βWT/−, plain green). Chimeric fusion of α-catenin to Ecad is sufficient to rescue the strength of single Ecad/Ecad bonds, (βWT/−(KD)EC-α, threaded blue), and (β−/Δ45EC-α). Loss of α-catenin leads to significantly weaker bonds (βWT/−-α-cat(KD, light blue). Results depict one-way ANOVA variance analysis using Bonferroni’s multiple comparison test, with ∗∗∗ implying α = 0.05 (95% confidence intervals). n > 140 for each case; number of independent experiments = 3 or more. (B) Strengthening of single Ecad/Ecad bonds over time depends on the state of β-catenin. For cells expressing WT β-catenin (βWT/−, green bars), Ecad/Ecad bonds strengthened over 300 ms, whereas for cells depleted of β-catenin (blue bars), Ecad/Ecad bonds weakened overt 300 ms (p = 0.0064; n = minimum 140 cells, one-way ANOVA analysis using Bonferroni’s multiple-comparison test with α = 0.05). Cells expressing Ecad-α-catenin chimera (β−/Δ45EC-α, orange bars), cells expressing mutant β-catenin (β−/Δ45, red bars), and parental HCT116 cells did not exhibit any time-dependent behavior. (C) Illustration showing the state of β-catenin when stronger or weaker E-cad/Ecad bonds are formed. (D) Thermodynamic analysis of rupture-force distribution based on the fit of bond strength distribution by the Hummer-Dudko model (left panel (20),) give rise to computation of transition length (x∗) and activation energy (Ea). Fit shows that (E) transition length increases and (F) free energy of activation decrease significantly for mutated or depleted β-catenin. ∗∗∗ Designates p < 0.001, unpaired Student’s t-test. A minimum of 140 ruptures were analyzed for each case, with N > 3. (G) In parental HCT116 colon-carcinoma cells, heterozygous expression of both WT β-catenin and mutated β-catenin leads to weakened Ecad/Ecad bonds between adjoining cells (p = 0.0072; n >140 cells each, one-way ANOVA analysis using Bonferroni’s multiple-comparison test with α = 0.05). Genetic depletion (shRNAi) of both β-catenin and known β-catenin kinase GSK3β has the same effect. n.s: not significant. (H) In CHO cells exogenously expressing Ecad, expression of S45 deletion-mutated β-catenin leads to the formation of weaker Ecad/Ecad bonds (p = 0.0093; n >140 cells each, one-way ANOVA analysis using Bonferroni’s multiple-comparison test with α = 0.05), whereas expression of phosphomimetic S > D mutated β-catenin induces no significant (n.s.) weakening of Ecad/Ecad bonds. Immunoprecipitation results (I) exhibit no significant change in Ecad/β-catenin binding affinity in CHO cells expressing ΔS45 mutant β-catenin. All error bars designate SEM.
