Figure 3.
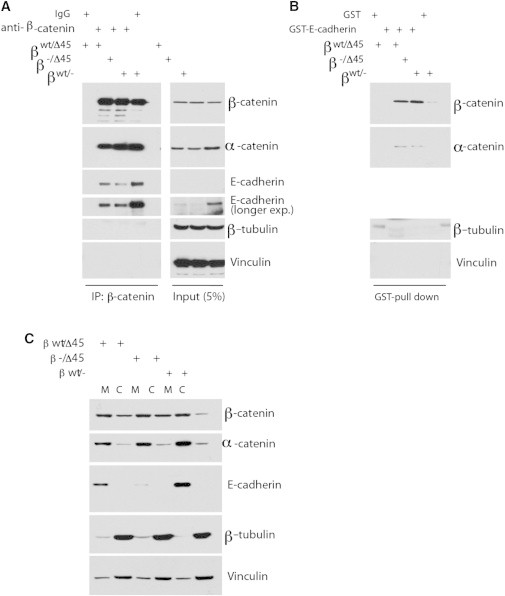
Affinity of WT and mutant β-catenin to E-cad remains unaltered. (A) β-catenin mutant binds to Ecad similarly to WT. Immunoprecipitation was performed using β-catenin antibody or mouse IgG charged protein G beads and cell lysates from HCT-derived cells as described in Methods, Western blotted as indicated. (B). Bacterially ecto-expressed E-cad cytoplasmic tail binds to β-catenin mutant. Bacterially expressed GST or GST-Ecad proteins were charged to Glutathione-agrose beads, pulldowns were performed as described and Western blotted. (C) Less cytosolic (lane C) β-catenin in WT/− than in mutant cells, as compared to membrane-bound β-catenin (lane M). Cells were fractionated and Western blotted.
