Abstract
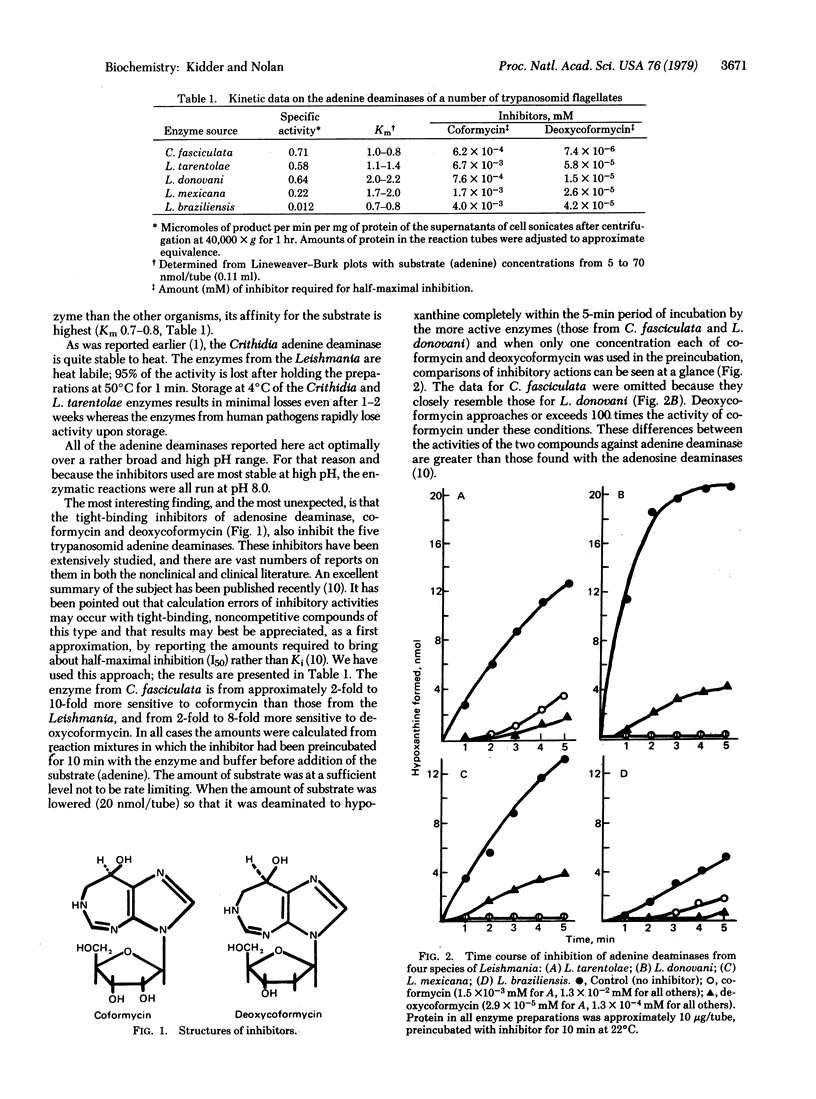
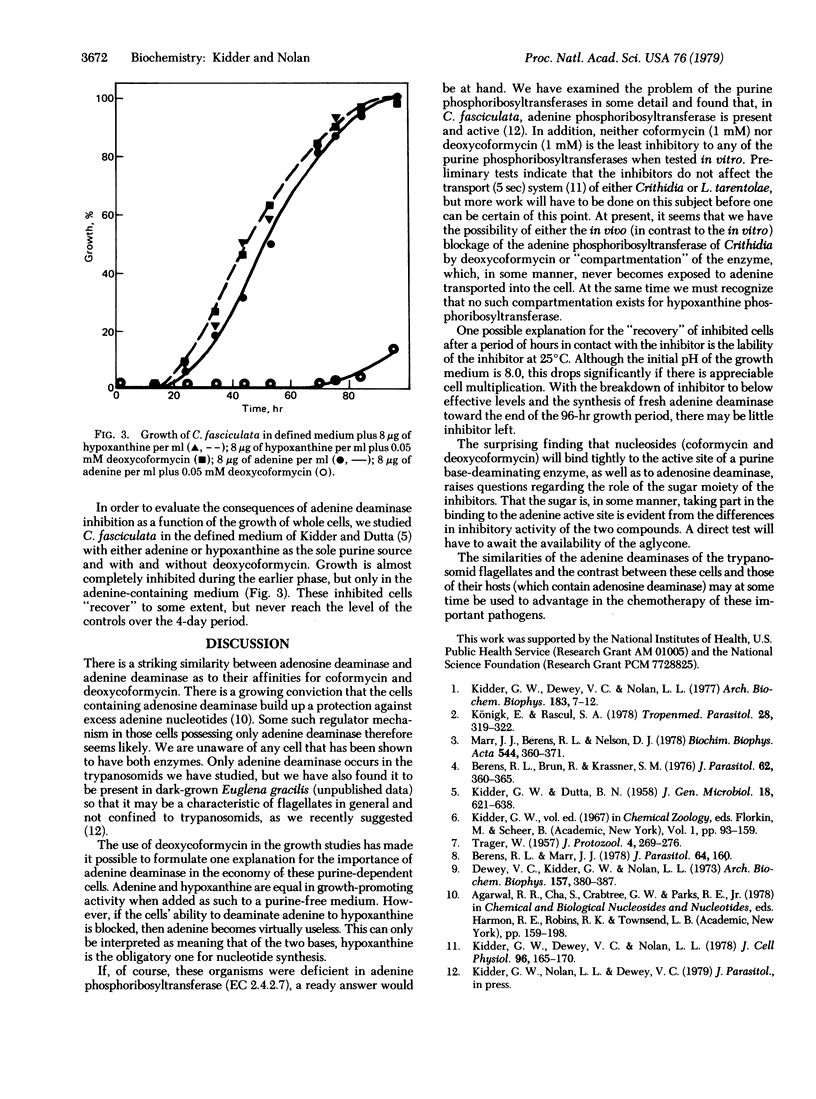
Adenine aminohydrolase (EC 3.5.4.2) from four species of Leishmania and from Crithidia fasciculata was examined for specific activities, affinity for substrate (adenine), and stability to heat. All were found to be strongly and non-competitively inhibited by both coformycin and deoxycoformycin, two tight-binding inhibitors of adenosine deaminase (adenosine aminohydrolase, EC 3.5.4.4). Deoxycoformycin is the more potent inhibitor of the two. Neither inhibitor was active against the purine phosphoribosyltransferases. When deoxycoformycin was added to the defined growth medium containing hypoxanthine as the purine source, the growth of C. fasciculata was unaffected, but when adenine was the purine source for the organism, severe inhibition resulted. This implies that hypoxanthine is the obligatory base for nucleotide synthesis and that the adenine phosphoribosyltransferase (AMP:pyrophosphate phosphoribosyltransferase, EC 2.4.2.7) is, in some manner,idenied access to exogenous substrate.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berens R. L., Brun R., Krassner S. M. A simple monophasic medium for axenic culture of hemoflagellates. J Parasitol. 1976 Jun;62(3):360–365. [PubMed] [Google Scholar]
- Berens R. L., Marr J. J. An easily prepared defined medium for cultivation of Leishmania donovani promastigotes. J Parasitol. 1978 Feb;64(1):160–160. [PubMed] [Google Scholar]
- Dewey V., Kidder G. W. Partial purification and properties of a nucleoside hydrolase from Crithidia. Arch Biochem Biophys. 1973 Aug;157(2):380–387. doi: 10.1016/0003-9861(73)90653-x. [DOI] [PubMed] [Google Scholar]
- KIDDER G. W., DUTTA B. N. The growth and nutrition of Crithidia fasciculata. J Gen Microbiol. 1958 Jun;18(3):621–638. doi: 10.1099/00221287-18-3-621. [DOI] [PubMed] [Google Scholar]
- Kidder G. W., Dewey V. C., Nolan L. L. Adenine deaminase of a eukaryotic animal cell, Crithidia fasciculata. Arch Biochem Biophys. 1977 Sep;183(1):7–12. doi: 10.1016/0003-9861(77)90412-x. [DOI] [PubMed] [Google Scholar]
- Kidder G. W., Dewey V. C., Nolan L. L. Transport and accumulation of purine bases by Crithidia fasciculata. J Cell Physiol. 1978 Aug;96(2):165–170. doi: 10.1002/jcp.1040960205. [DOI] [PubMed] [Google Scholar]
- Königk E., Rasoul S. A. Catabolism of adenosine 5'-monophosphate in promastigotes of Leishmania tropica. Tropenmed Parasitol. 1978 Sep;29(3):319–322. [PubMed] [Google Scholar]
- Marr J. J., Berens R. L., Nelson D. J. Purine metabolism in Leishmania donovani and Leishmania braziliensis. Biochim Biophys Acta. 1978 Dec 1;544(2):360–371. doi: 10.1016/0304-4165(78)90104-6. [DOI] [PubMed] [Google Scholar]


