Abstract
Brain activation is adaptive to task difficulty and practice. We used functional MRI to map brain systems activated by an object-location learning task in 24 healthy elderly volunteers each scanned following placebo and two of four active drugs studied. We distinguished a fronto-striatal system adaptive to difficulty from a posterior system adaptive to practice. Fronto-striatal response to increased cognitive load was significantly attenuated by scopolamine, sulpiride and methylphenidate; practice effects were not modulated by these drugs but were enhanced by diazepam. We also found enhancement by methylphenidate, and attenuation by sulpiride, of load response in premotor, cingulate and parietal regions comprising a spatial attention network. Difficulty and practice evoke anatomically and pharmacologically dissociable brain activation dynamics, which are probably mediated by different neurotransmitter systems in humans.
Introduction
Practice makes perfect: repeated performance of a task tends to make it less difficult to do, or easier to do well. Thus practice and difficulty can be correlated or confounded behaviorally. Likewise functional neuroimaging has shown that practice and difficulty can have negatively correlated effects on brain activation: both unpracticed and difficult tasks tend to evoke relatively increased brain activation. Neurocognitive load response describes the well-replicated observation that increased task difficulty or cognitive load is often associated with increased brain activation as measured by positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) in a variety of experimental settings (Grasby et al., 1994; Just et al., 1996; Braver et al., 1997; Jonides et al., 1997; Carpenter et al., 1999; Honey et al., 2000; Ng et al., 2001). Practice effects broadly describe another frequently reported observation: namely, decreased brain activation as a correlate of increased familiarity or repeated performance of a cognitive task or stimulus (Buckner et al., 1998; Büchel et al., 1999; Naccache and Dehaene, 2001). This phenomenon was described as repetition suppression, based on single unit recordings from inferior temporal cortex in monkeys (Desimone, 1996), and has since been observed in ventral occipital and other brain regions and over longer time-scales of stimulus repetition in humans (Henson et al., 2000). Complementary modes of brain adaptivity to task repetition, i.e. increased or consolidated activation as a function of increased familiarity, have also been reported especially as a correlate of motor or visual perceptual skill acquisition (Karni et al., 1998; Poldrack, 2001).
Since an unpracticed task is generally more difficult than a practiced task, it is possible to imagine that dynamic changes in brain activation evoked by these two factors might represent different aspects of a unitary control process, or attentional economy, for regulating the supply of metabolically costly brain activation in relation to variable demand for cognitive processing (Laughlin et al., 1998). By this account, one might expect that load response and practice dynamics were expressed in the same anatomical systems in a given experimental paradigm and/or that they were modulated identically by a range of psychoactive drugs selectively acting at various receptors.
We have tested these hypothetical predictions by a randomized, placebo-controlled study of the effects of methylphenidate, sulpiride, diazepam and scopolamine on brain activation measured with fMRI in 24 healthy, elderly volunteers during performance of an object-location learning task. Each subject was randomly assigned to one of two treatment groups (A and B) and scanned on three occasions following treatment in counter-balanced order with placebo, methylphenidate or sulpiride (Group A) or placebo, diazepam or scopolamine (Group B).
Besides its specific hypothesis-testing objectives, this design was also strategically motivated by the possibility of using pharmacological fMRI to map normal age-related changes in human neurotransmitter systems in future studies. Age-related declines of executive and memory functions in non-human primates, for example, have been linked to marked changes in dopamine receptor density and dopaminergic drug effects on brain function (Wenk et al., 1989; Arnsten et al., 1995). These and other data raise the possibility that normative developmental changes in human cognitive functions over the course of the life-cycle, as well as mild cognitive impairment and some dementia syndromes, might be explicable in terms of the neurocognitive consequences of longitudinal change in neurotransmitter systems. To evaluate this possibility empirically, we need a method of imaging transmitter-specific effects on neurocognitive function that is safe enough to be used repeatedly in normal participants and patients of all ages. Pharmacological fMRI could potentially meet those requirements (Zhang et al., 2001) but we currently know little about its feasibility, or sensitivity to detect drug effects on neurocognitive function, in elderly human participants. Older participants have shown significantly attenuated activation of episodic and working memory systems compared with younger participants (Grady et al., 1999; Rypma and D’Esposito, 2000) and blood oxygen level dependent (BOLD) signals measured by fMRI may generally be lower amplitude and noisier in older participants (D’Esposito et al., 1999). Additionally, there is likely to be more variability of brain structure between older participants (Thompson et al., 1998), which might result in greater image registration error and mitigate the sensitivity to detect treatment effects on activation measured in a standard coordinate system. All of these factors would be expected to curtail the power of fMRI in future studies of age-related change in psychopharmacological drug effects. It therefore seemed timely and generally useful to assess the method empirically in an elderly sample. Four aspects of design and analysis of the study seemed particularly important for enhancing its power.
First, we recognized that there is considerable idiosyncratic inter-subject variability in brain activation measured using fMRI and detection of drug effects in this context is likely to benefit from a within-subject or repeated measures design in which each participant is scanned following placebo and one or more active compounds. Furthermore, we considered that it was unreasonable to expect elderly participants to attend for scanning on more than three separate occasions, which limited the number of active compounds administered to two for each subject.
Second, we selected drugs and paired them for administration in the same group of participants principally on the grounds that the drugs in each treatment group should be instructively comparable with each other. Partly because of the primate studies on age-related change in dopamine systems alluded to earlier, we were interested in the effects of dopaminergic drugs on human learning and memory. We therefore combined sulpiride, a dopamine D2 receptor antagonist, with methylphenidate, an indirect monoaminergic agonist, in treatment Group A. We anticipated that the opposing effects of these two drugs at the level of dopaminergic synapses might be reflected in opposing effects on function of large-scale neurocognitive networks. Likewise, we were interested to compare and contrast the physiological effects of scopolamine and diazepam. Scopolamine is an antagonist at muscarinic acetylcholine receptors and diazepam is an agonist at γ-aminobutyric acid (GABA) receptors; however both drugs cause a reversible amnestic syndrome in humans and we wanted to know whether or not this behavioral effect in common reflected shared effects on neurocognitive function. Scopolamine and diazepam were therefore combined in treatment Group B. It was also critically important that the drugs used in this study should be safe and tolerable by elderly participants. In this respect it was highly advantageous that each of these four drugs has been widely used and well-tolerated in clinical practice – sulpiride as an antipsychotic, methylphenidate as a stimulant, diazepam as a sedative/hypnotic and scopolamine as a surgical premedication.
Third, we were concerned to use a highly efficient activation paradigm so that cognitively induced signal could be estimated with minimal error. It is known that blocked periodic designs are considerably more efficient than event-related designs for fMRI (Friston et al., 1999). We therefore used a periodic contrast between blocked baseline and activation conditions to generate an efficiently estimated periodic signal. We also varied the difficulty of the object-location learning trials between epochs of trials, presenting four epochs of trials at each of two levels of difficulty. At the more difficult (four-item) level, subjects were briefly presented with an array of four highly discriminable symbols located around a central crosshair and, in each trial, immediately tested on the location of one symbol randomly selected from the array. At the less difficult (two-item) level, subjects were repeatedly tested in the same way using an array of two symbols. Each level of the task was presented in four 24 s epochs each comprising four 6 s trials. This design allowed us separately to model blocked effects of task difficulty and practice, as well as the periodic contrast between baseline and (all) activation conditions.
Fourth, we wanted to retain an acceptable level of control over false positive (type 1) errors in brain mapping without imposing unacceptably high levels of false negative (type 2) error in the context of low signal : noise. To do this we adopted two complementary strategies. We tested drug treatment effects at a regional level, using prior analysis of data acquired following placebo treatment (n = 24) to identify ‘regions of interest’, i.e. brain systems that normally or typically demonstrated adaptivity to difficulty and/or practice. More comprehensively, we also tested between-treatment differences at the level of suprathreshold voxel clusters (n = 12 in each group) to identify systems of ‘atypical’ or drug-related adaptivity. Cluster-level statistics are generally more sensitive than voxel statistics to effects distributed over a region or local neighborhood of voxels; there are also usually one or two orders of magnitude fewer cluster-level statistics than there are voxel statistics to test in a whole brain map. The greater sensitivity and smaller search volume entailed by cluster-level inference are especially attractive, as in this case, when the power of the design may be jeopardized by other factors. Since cluster-level statistics in fMRI are generally not well approximated by asymptotic theory, we used a nonparametric mode of inference based on computationally intensive data resampling for hypothesis testing (Bullmore et al., 1999b).
Materials and Methods
Sample and Study Design
Twenty four, right-handed, healthy, elderly volunteers were recruited: 9 male, 15 female; mean age = 72.4 years, age range = 61–80 years. All participants had a normal medical examination and supplementary investigations including ECG and urine drug screen to exclude any neurological, cardiovascular or psychiatric disorder, any contraindication to MRI, or any undeclared drug use. All participants provided informed consent in writing. The study was approved by the Ethics (Research) Committee of the Bethlem Royal & Maudsley NHS Trust.
We used a randomized, single-blind, placebo-controlled design; for ethical and clinical reasons one member of the study team present during scanning was not blind to treatment. Participants were scanned using fMRI in three separate sessions at 14 day intervals. To habituate participants to the MR scanning environment, and to acquire structural MRI data, participants were also briefly scanned during a preliminary assessment before they started the fMRI study.
Half of the sample (Group A; n = 12; four male, eight female; mean age = 69.75 years) was randomly assigned to receive one of the following oral treatments before each scanning session: placebo, sulpiride 400 mg, methylphenidate 20 mg. The other half of the sample (Group B; n = 12; five male, seven female; mean age = 75 years) was assigned to receive one of the following treatments before each scanning session: placebo, scopolamine, diazepam. Scopolamine was administered subcutaneously at an average dose of 0.35 mg; six participants received 0.4 mg and six received 0.3 mg. Diazepam 5 mg was administered orally. To control for mode of administration, half of the participants in Group B had oral placebo and half had a subcutaneous injection of saline.
The order of treatments was counterbalanced across subjects so that each of six possible treatment orders was used twice in each group of 12 subjects.
Because these four active compounds have different pharmacokinetic profiles, they were administered at different times before scanning to ensure effective and stable plasma concentrations during fMRI: sulpiride and diazepam were given 180 min prior to scanning; methylphenidate, scopolamine and placebo were given 90 min before scanning. All drugs were well tolerated at these doses and all participants successfully completed the scanning protocol.
fMRI Data Acquisition
Gradient-echo echoplanar imaging (EPI) data were acquired at 1.5 T using a GE LX-NV/CV system equipped with ultra-fast SR150 field gradients allowing a maximum gradient amplitude of 40 mT/m (General Electric, Milwaukee WI, USA). For each activation experiment, 16 near-axial slices of functional MRI data were acquired with the following parameters: repetition time (TR) = 2000 ms; echo time (TE) = 40 ms; flip angle = 70°; slice thickness = 7 mm; interslice gap = 0.7 mm; matrix size = 64 × 64. To facilitate later registration of fMRI data in standard space, a higher resolution EPI dataset comprising 43 near-axial slices was also acquired with the following parameters: TR = 6000 ms; TE = 40 ms; inversion time (TI) = 1500 ms; flip angle = 90°; slice thickness = 3 mm; interslice gap = 0.3 mm; matrix size = 128 by 128.
Object-location Learning
Object-location learning trials (see Fig. 1) were presented in 24 s epochs, alternating with 24 s epochs of crosshair fixation; this cycle was repeated eight times so the total duration of the experiment was 6 min 24 s = 192 images. Cognitive load was manipulated by presenting learning trials at one of two possible levels of difficulty in randomized order within each set of two consecutive activation epochs. In an easy trial, a set of two stimuli (highly discriminable colored shapes) were shown for 1500 ms either side (left and right) of a central crosshair, followed by 500 ms of central crosshair alone, followed by central presentation of one of the two original stimuli for 2000 ms. The subject was trained to move a joystick in the direction of the location (left or right) originally occupied by the central stimulus. A difficult trial was exactly the same except that a set of four stimuli were initially arrayed around the central crosshair. For each level of difficulty, the same set of stimuli was repeatedly presented over all trials. For both levels of difficulty, trial duration was 6 s, subjects were immediately informed by visual feedback whether the response to each trial was correct or incorrect, and accuracy and reaction time (RT) were monitored by joystick movements during scanning. Note that this design does not allow us to disambiguate effects of stimulus repetition from effects of repeated task performance or skill acquisition on brain activation. We therefore refer to the correlates of repeated presentation of the task non-specifically as practice or familiarity effects. It is also noteworthy that the experimental paradigm provides immediate visual feedback to the participants, which distinguishes this task from a classical paired associate learning task and may explain the greater salience of fronto-striatal than hippocampal activation observed in the imaging data (Poldrack et al., 2001).
Figure 1.
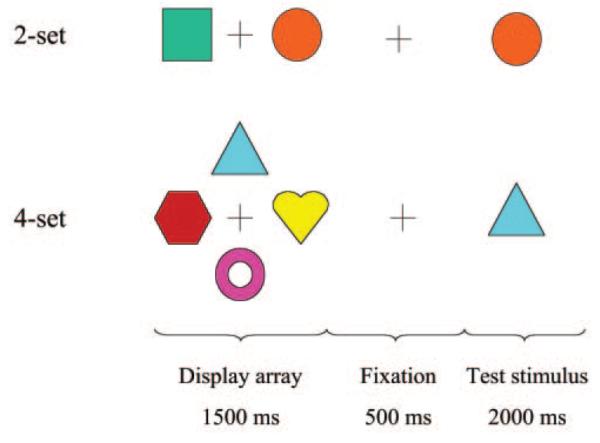
Schematic of object-location learning trials at two levels of difficulty, two-item learning (top) and four-item learning (bottom). In each trial, an array of easily discriminable shapes is presented for 1500 ms around a central crosshair, followed by 500 ms of fixation on a central crosshair, followed by central presentation of one of the shapes as a test stimulus for 2000 ms. Subjects moved a joystick to indicate the position of the test stimulus in the original array and received immediate visual feedback concerning the correctness of their decisions. The same array of shapes was repeatedly presented in four 24 s epochs at each level of difficulty.
Imaging Data Analysis
Following correction of head movement-related effects in the fMRI time series (Bullmore et al., 1999a), linear regression was used to estimate experimentally induced signal changes (Bullmore et al., 2001). Regression analysis modeled three mutually orthogonal aspects of brain activation at each voxel: (i) a periodic contrast between baseline and activation conditions which characterized the response to object-location learning ‘on average’ over all blocks; (ii) a contrast between the two levels of difficulty of the activation condition which characterized cognitive load response; and (iii) a linear contrast between epochs coded according to their order of presentation, which was sensitive to effects of task familiarity. [We will refer to the first (second, third or fourth) pair of easy and difficult epochs as the first (second, third or fourth) block of trials; and therefore the results of this analysis will later be described as sensitive to between-block repetition effects.] Prior to model fitting, each contrast was convolved by a pair of Poisson kernels (λ = 4 or 8 s) to model locally variable hemodynamic response functions. Statistic maps representing each of these three standardized effects for each individual under each drug treatment were registered in the standard space of Talairach and Tournoux (Talairach and Tournoux, 1988) by an affine transformation to a template image (Brammer et al., 1997).
For within-group analysis, activation maps indicating areas of significant response within each treatment condition were created by a permutation test of the median standardized effect at each voxel or suprathreshold voxel cluster (Brammer et al., 1997; Bullmore et al., 1999b). Cluster level analysis involved applying a preliminary probability threshold (P < 0.05) to the voxel statistic maps and setting all subthreshold voxels to zero, thus creating a set of suprathreshold voxel clusters that are spatially contiguous in 3D. The sum of suprathreshold voxel statistics or ‘mass’ of each cluster was then tested by a permutation test at each cluster (Bullmore et al., 1999b).
Between-group analysis was conducted in two ways. First, to investigate the effect of active drug treatments on normally or typically load-responsive brain systems, the areas that showed a significant effect of task difficulty in the fMRI data acquired following placebo were used as a ‘mask’ or ‘region-of-interest’ to extract regional mean effects of difficulty from each individual dataset under all treatment conditions. Significant differences between each active treatment and placebo were then identified in these regional data by paired t-tests. Second, to identify regions that showed a significant effect of drug treatment outside typically adaptive systems, a repeated measures analysis of variance (ANOVA) model including a single three-level factor for treatment was fitted at each intracerebral voxel to data acquired from subjects receiving the same set of active treatments (n = 12 in each group). Significant treatment effects were identified by a cluster-level permutation test, as described above, and further investigated post hoc by paired t-tests.
We have consistently adopted stringent levels of statistical significance for all the hypothesis tests reported on imaging data. For generic activation maps, one-tailed voxelwise probability of type 1 error P < 0.0001. At this size of test we expect less than one false positive test over all the voxels tested in each map. For all within-group and between-group ANOVAs conducted at cluster level, one-tailed clusterwise probability of type 1 error P ≤ 0.01. At this size of test we expect less than one false positive cluster over all the clusters tested in each ANOVA map.
Behavioral Data Analysis
ANOVA was also used to estimate the main effects of difficulty and familiarity, and the interaction between them, on behavioral data recorded during scanning following placebo (n = 24). Repeated measures ANOVA was used to estimate main effects of difficulty, familiarity and drug treatment, and the interactions between these factors, separately in each treatment group (n = 12).
Results
Behavioral Data
There were highly significant effects of both difficulty (number of items) and practice (block number) on latency of task performance during scanning after placebo treatment: main effect of number of items, F(1,23) = 100.4, P < 0.001; main effect of block number, F(3,21) = 10.79, P < 0.001. There were also significant effects of both difficulty and practice on accuracy of task performance measured during scanning after placebo: main effect of number of items, F(1,23) = 18.8, P < 0.001; main effect of block number, F(3,21) = 4.5, P = 0.014. As shown in Figure 2 and Table 1, difficulty tended to prolong RT and reduce accuracy whereas practice tended to reduce RT and improve accuracy. It is clear from these data that the behavioral effect of load was more salient than the effect of practice. The interaction between difficulty and practice was not significant for either latency or accuracy. Similar behavioral effects of both difficulty and practice were seen in both treatment groups analyzed separately. The main effect of drug treatment was not significant in either group. The interactive effects of drug × load and drug × block number were not significant for accuracy or latency of response in either treatment group.
Figure 2.
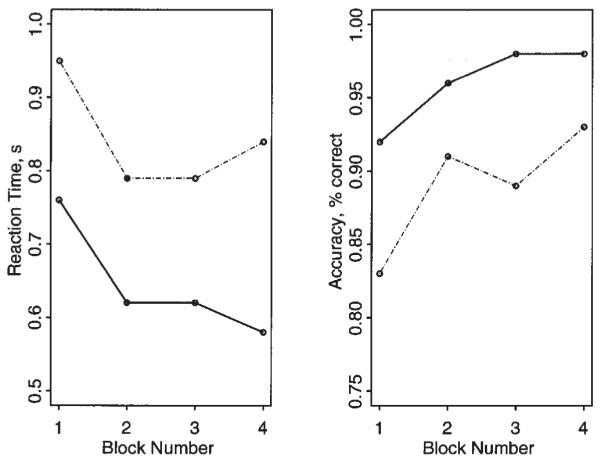
Behavioral effects of cognitive load (task difficulty) and repeated performance (task familiarity) following treatment with placebo (n = 24). Left panel: block number is plotted against RT for two-item object-location learning (solid line) and four-item object-location learning (broken line). Right panel: block number is plotted against accuracy (proportion of trials correct) for two-item object-location learning (solid line) and four-item object-location learning (broken line). Increased difficulty tends to prolong RT and impair accuracy, whereas increased familiarity has opposite, though less salient, effects on behavior. Load and practice effects were also significant in each treatment group considered separately; there was no significant effect of treatment in either group.
Table 1.
Behavioral data for performance on object-location learning task following placebo (n = 24; SDs in parentheses)
| Block no. | Two-item learning |
Four-item learning |
||
|---|---|---|---|---|
| RT (s) | Accuracy, % correct | RT (s) | Accuracy, % correct | |
| 1 | 0.76 (0.22) | 0.92 (0.18) | 0.95 (0.30) | 0.83 (0.19) |
| 2 | 0.62 (0.18) | 0.96 (0.11) | 0.79 (0.21) | 0.91 (0.16) |
| 3 | 0.62 (0.17) | 0.98 (0.10) | 0.79 (0.22) | 0.89 (0.18) |
| 4 | 0.58 (0.17) | 0.98 (0.10) | 0.84 (0.20) | 0.93 (0.18) |
Although treatments were administered in counterbalanced order within each group to obviate confounding effects of session order, we confirmed post hoc that there was no significant effect of session order, and no interactive effect of session order × block number, on accuracy or latency of response in both groups.
fMRI Data Acquired Following Placebo
All images acquired following placebo treatment (n = 24) were included in a within-group analysis of activation–baseline contrast, load response and familiarity effects. As expected due to the cognitively undemanding nature of the baseline condition, the average periodic response was significant in a widely distributed system including ventral and dorsal extrastriate cortical regions, bilateral prefrontal cortex, basal ganglia and cerebellum. Areas of significant load response were more circumscribed, including principally bilateral dorsal prefrontal cortex [approximate Brodmann areas (BA) 46, 10, 9, 8] extending posteriorly to lateral premotor (BA 6) and precentral (BA 4) regions in the right hemisphere; left anterior cingulate gyrus (BA 24, 32); right inferior frontal gyrus (BA 47); right insula/claustrum; right caudate nucleus and putamen; and right hippocampus and parahippocampal gyrus (BA 27, 30) (Fig. 3 and Table 2).
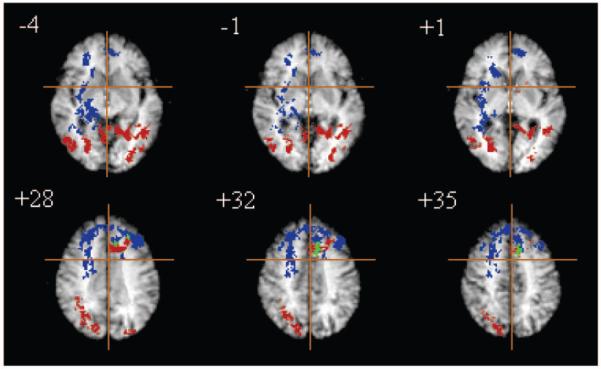
Figure 3.
Brain systems typically adaptive to task difficulty and task practice are anatomically dissociable. Functional MRI data acquired from 24 subjects following placebo treatment are summarized in selected slices of a generic brain activation map; the right side of the brain is shown on the left side of each slice; the crosshair locates the origin of x and y axes of Talairach space in the plane of each slice; the z coordinate of each slice in millimeters above or below the intercommissural line is also shown. Blue voxels are adaptive to difficulty, red voxels are adaptive to practice, and green voxels are adaptive to both difficulty and practice. Significance testing is by a permutation test at suprathreshold voxel cluster level: clusterwise P < 0.01. At this size of test, we expect less than one false positive cluster per map.
Table 2.
Typical adaptation dynamics: brain regions demonstrating significant load response and practice effects in the object-location learning task following placebo treatment (n = 24)
| Region (approx. Brodmann area) | Talairach coordinates of 2-D cluster centroid (mm) |
Cluster area (voxels) |
||
|---|---|---|---|---|
| x | y | z | ||
| (A) Main locations of load response (increased signal with increased load) | ||||
| Insula | 32 | 8 | −12 | 15 |
| 34 | 6 | −8 | 11 | |
| 32 | −6 | −8 | 16 | |
| Parahippocampal gyrus | 20 | −31 | −8 | 11 |
| (BA 28, 35) | 22 | −30 | −4 | 103 |
| Anterior cingulate gyrus | −5 | 41 | −4 | 28 |
| (BA 24, 32) | −11 | 41 | 1 | 32 |
| −11 | 40 | 4 | 33 | |
| −9 | 37 | 8 | 58 | |
| −5 | 40 | 12 | 48 | |
| −6 | 39 | 16 | 118 | |
| 10 | 37 | 20 | 107 | |
| 16 | 32 | 24 | 140 | |
| 18 | 31 | 28 | 117 | |
| 8 | 19 | 32 | 277 | |
| Inferior/middle frontal gyrus | 30 | 0 | 20 | 150 |
| (BA 44, 46) | −38 | 21 | 20 | 21 |
| Caudate nucleus | 18 | 17 | 4 | 48 |
| 17 | 17 | 8 | 45 | |
| Putamen | 28 | −14 | 12 | 56 |
| (B) Main locations of practice effects (habituation of signal as a linear function of between-block repetition) | ||||
| Cerebellum | 21 | −58 | −28 | 188 |
| 21 | −56 | −24 | 176 | |
| −6 | −53 | −20 | 130 | |
| Fusiform gyrus | 31 | −38 | −16 | 33 |
| (BA 19, 20) | 43 | −67 | −12 | 50 |
| Lingual gyrus | −18 | −87 | −12 | 36 |
| (BA 18, 19) | −29 | −55 | −4 | 106 |
| Inferior temporal gyrus | 45 | −67 | −8 | 51 |
| (BA 37) | ||||
| Parahippocampal gyrus | −18 | −52 | 1 | 46 |
| 3 | −35 | 4 | 7 | |
| Thalamus | −10 | −10 | 8 | 92 |
| Anterior cingulate gyrus | −15 | 15 | 24 | 41 |
| (BA 24, 32) | −14 | 17 | 28 | 78 |
| −11 | 15 | 32 | 80 | |
| Superior occipital gyrus | 26 | 80 | 24 | 59 |
| (BA 19) | 29 | −69 | 28 | 37 |
| 28 | −67 | 32 | 30 | |
| Inferior parietal lobule | 34 | −67 | 40 | 39 |
| (BA 7, 40) | 31 | −70 | 45 | 45 |
This predominantly frontal load-responsive system was almost entirely distinct anatomically from the set of regions which showed a significant change in activation related to task practice: bilateral cerebellar hemispheres and cerebellar vermis; right fusiform gyrus (BA 19) extending to inferior temporal cortex (BA 37) laterally; right anterior hippocampus; left posterior hippocampus, parahippocampal gyrus and retrosplenial cortex; left thalamus and caudate nucleus; and bilateral dorsal extrastriate cortex (BA 19) extending to inferior parietal areas (BA 39, 40) superiorly; Figure 3 and Table 2. The only brain region that showed significant effects of both task difficulty and practice was located in the left anterior cingulate gyrus (BA 32) (see Fig. 3).
Drug Effects on Normally Adaptive Systems
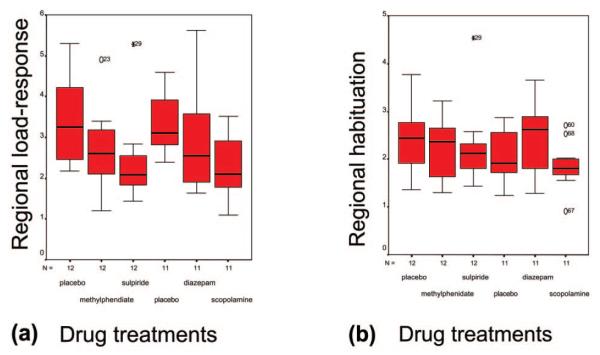
The effects of active drug treatment on brain systems which showed significant adaptation to difficulty and practice in the placebo-treated data were investigated at regional level. Normal load responsivity was significantly attenuated by scopolamine [paired t-test, t(11) = 3.74, P = 0.004], sulpiride [paired t-test, t(11) = 2.82, P = 0.017] and methylphenidate [paired t-test, t(11) = 2.38, P = 0.036] but not significantly affected by diazepam. There was no significant effect of any active compound on systems normally adaptive to practice. These results are summarized graphically in Figure 4.
Figure 4.
Box-plots of (a) load response and (b) practice effects in typically adaptive brain systems defined as regions-of-interest by a prior analysis of data acquired following placebo. In a predominantly fronto-striatal system (a), sulpiride, methylphenidate and scopolamine (but not diazepam) all caused significant attenuation of normal load response. None of these drugs significantly modulated adaptivity to between-block repetition in a more posterior system (b). See Table 2 for anatomical details of these brain systems.
Drug Effects on Atypical Activation and Load response
In treatment Group A (methylphenidate and sulpiride), there were significant between-treatment differences in periodic activation in right anterior cingulate gyrus and dorsolateral prefrontal cortex, right caudate nucleus, and left ventral occipital and medial temporal lobe structures including fusiform and lingual gyri, parahippocampal gyrus and hippocampus. Post hoc analysis demonstrated that this was due to a significant enhancement of periodic response by methylphenidate [paired t-test, t(11) = 3.52, P = 0.005]. There was a trend for sulpiride to have the opposite (attenuating) effect on periodic activation but this was not quite statistically significant [paired t-test, t(11) = –2.02, P = 0.069]. The anatomy of this effect is illustrated by selected slices of the corresponding ANOVA map in Figure 5 (top); details are provided in Table 3.
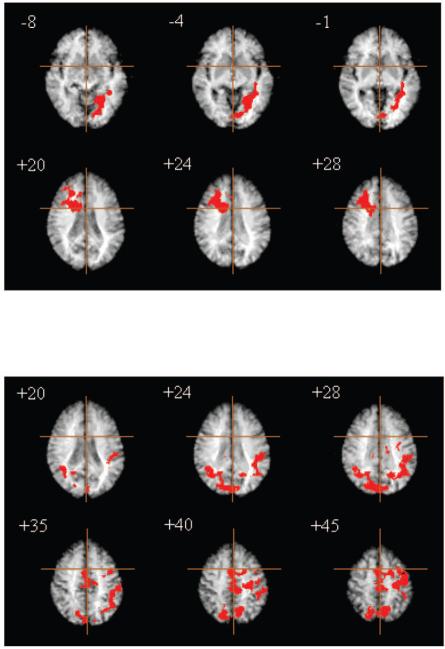
Figure 5.
Effect of dopaminergic drugs (methylphenidate and sulpiride) on atypical brain activation; treatment Group A, n = 12. Top, right prefrontal and left ventral occipital regions that demonstrated significant enhancement of periodic activation by methylphenidate; bottom, medial posterior parietal, anterior cingulate/SMA, left lateral premotor and temporoparietal regions that demonstrated significant enhancement of load response by methylphenidate and significant attenuation of load response by sulpiride. Significance testing is by a permutation test at suprathreshold voxel cluster level: clusterwise P < 0.01. At this size of test, we expect less than one false positive cluster per map.
Table 3.
Atypical adaptation dynamics: brain regions demonstrating significant effects of drug treatment (methylphenidate and sulpiride) on periodic activation and load response in the object-location learning task (Group A; n = 12)
| Region (approx. Brodmann area) | Talairach coordinates of 2-D cluster centroid (mm) |
Cluster area (voxels) |
||
|---|---|---|---|---|
| x | y | z | ||
| (A) Main locations of treatment effect on periodic activation | ||||
| Parahippocampal gyrus | −38 | −43 | −8 | 20 |
| (BA 35, 36) | ||||
| Fusiform gyrus | −21 | −63 | −8 | 132 |
| (BA 19) | ||||
| Lingual gyrus | −23 | −60 | −4 | 209 |
| (BA 19) | −31 | −51 | −1 | 107 |
| Caudate nucleus | 17 | 16 | 16 | 206 |
| Anterior cingulate gyrus | 24 | 8 | 28 | 179 |
| (BA 24, 32) | ||||
| Inferior frontal gyrus | 29 | 9 | 32 | 55 |
| (BA 44) | ||||
| (B) Main locations of treatment effect on load response | ||||
| Inferior parietal lobule | −42 | −44 | 24 | 115 |
| (BA 39, 40) | −35 | −52 | 28 | 182 |
| −36 | −53 | 32 | 183 | |
| −41 | −48 | 35 | 132 | |
| Cuneus | 17 | −74 | 28 | 232 |
| (BA 19) | 8 | −82 | 32 | 122 |
| 9 | −80 | 35 | 56 | |
| Premotor/precentral cortex | −38 | −4 | 35 | 24 |
| (BA 4, 6) | −34 | −18 | 45 | 157 |
| −37 | −26 | 50 | 97 | |
| Cingulate gyrus/SMA | 0 | −18 | 35 | 108 |
| (BA 24, 6m) | −3 | −18 | 40 | 186 |
| −3 | −16 | 45 | 180 | |
| 1 | 5 | 50 | 99 | |
| 3 | 5 | 55 | 101 | |
| Precuneus | −9 | −70 | 40 | 63 |
| (BA 7) | 3 | −71 | 45 | 157 |
In Group A, there were also significant between-treatment differences in load response in mid-dorsal anterior cingulate gyrus (BA 23, 24) extending superiorly to medial premotor cortex [BA 6; supplementary motor area (SMA)]; left lateral premotor and precentral cortex (BA 6, 4); bilateral cuneus (BA 19) extending dorsally to medial posterior parietal cortex (BA 7); and left temporo-parietal cortex including areas of superior temporal gyrus (BA 22), supramarginal and angular gyri (BA 39, 40). Post hoc analysis demonstrated that this was due to a significant enhancement of load response in these regions by methylphenidate [paired t-test, t(11) = 4.84, P = 0.001] and to a significant attenuation of load response by sulpiride [paired t-test, t(11) = –5.05, P < 0.001]. The anatomy of this effect is illustrated by selected slices of the corresponding ANOVA map in Figure 5 (bottom); details are provided in Table 3.
In treatment Group B (scopolamine and diazepam), there were significant between-treatment differences in periodic activation in bilateral cerebellum; midbrain; right lateral occipital cortex (BA 19) extending anteriorly to inferior temporal cortex (BA 37) and dorsally to cuneus (BA 19) and medial posterior parietal cortex (BA 7); bilateral inferior parietal lobule (BA 40); and mid-dorsal anterior cingulate gyrus (BA 23, 24). Post hoc analysis demonstrated that this was due to significant enhancement of periodic activation by diazepam [paired t-test, t(11) = 3.45, P = 0.006] and less salient attenuation of periodic activation by scopolamine (paired t-test, t = –2.80, P = 0.019).
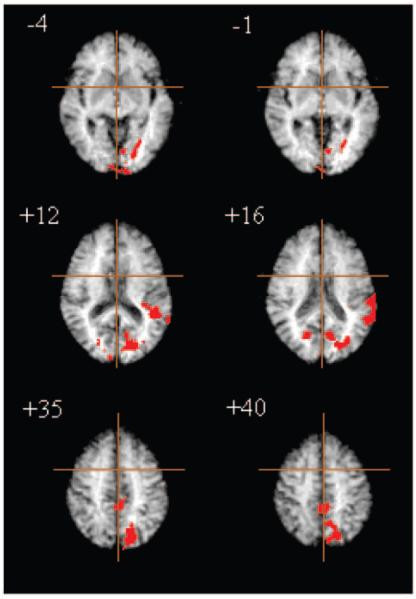
In Group B, there were also significant between-group differences in atypical load-response and practice effects. Treatment effects on atypical load response were located predominantly in bilateral dorsal prefrontal cortex (BA 45, 46), bilateral inferior parietal lobule (BA 39, 40), bilateral cuadate nucleus and putamen, and cuneus (BA 18, 19) extending dorsally to medial posterior parietal cortex (BA 7). Treatment effects on practice were located more exclusively in posterior regions including left lingual gyrus (BA 18, 19) and left superior temporal gyrus (BA 22), bilateral cuneus (BA 19) and precuneus (BA 7). In both cases, post hoc analysis demonstrated that these effects were due to a significant enhancement by diazepam of load response [paired t-test, t(11) = 6.55, P < 0.001] and practice effects (paired t-test, t = 2.841, P = 0.018). There were no significant effects of scopolamine on load response or practice effects outside normally adaptive systems. The anatomy of Group B drug effects on atypical adaptivity to practice is illustrated by selected slices of the corresponding ANOVA map in Figure 6.
Figure 6.
Effects of diazepam on atypical brain activation; treatment Group B (n = 12). Areas of ventral and dorsal occipital cortex and left superior temporal cortex demonstrated significant enhancement of practice effects following diazepam. Significance testing is by a permutation test at suprathreshold voxel cluster level: clusterwise P < 0.01. At this size of test, we expect less than one false positive cluster per map.
Discussion
Drug Effects on Typical Adaptivity to Difficulty and Practice
Although difficulty and practice can be confounded behaviorally, as in the familiar case of an unpracticed task being more difficult, these results clearly show that the brain activation dynamics evoked by task difficulty and practice are separable. We have shown that human brain systems adaptive to difficulty and repeated performance of an object-location learning task are almost entirely disjoint anatomically. Load response was located in a predominantly fronto-striatal system, whereas practice effects on brain function were located in a posterior system comprising cerebellum, medial temporal lobe structures and several regions of extrastriate occipital and inferior temporal cortex. Many of these regions have previously been shown to demonstrate practice effects such as repetition suppression in humans and monkeys (Desimone, 1996; Büchel et al., 1999). The only brain region that was significantly adaptive to both difficulty and practice was located in the left anterior cingulate gyrus.
In addition to these marked anatomical differences between load response and practice effects, we have also demonstrated that these two modes of dynamic change in brain activation are pharmacologically distinguishable. Normal load-response systems were significantly attenuated by scopolamine, sulpiride and methylphenidate, but not by diazepam; whereas systems normally adaptive to practice were not significantly affected by any of these compounds. This pattern of results suggests that neurocognitive load response in fronto-striatal systems may normally be mediated by cholinergic and dopaminergic neurotransmitter systems or, at least, that load response is preferentially sensitive to cholinergic and dopaminergic modulation. There have been no previous pharmacological MRI studies explicitly addressing the susceptibility of load response dynamics to various drug challenges in humans. The closest prior work is by Mattay et al. (Mattay et al., 2000) who demonstrated a significant interaction between dextroamphetamine and level of difficulty of a working memory task on prefrontal cortical activation. However, there is relatively abundant evidence for the importance of dopaminergic and cholinergic systems in attentional modulation of prefrontal and anterior cingulate areas in rats (Ragozzino, 2000) and monkeys (Blokland, 1996; Robbins, 2000). We suggest that our findings are compatible with this precedent in animal models and indicate that attentional modulation of fronto-striatal circuits in humans may also be critically dependent on cholinergic and dopaminergic transmitter systems.
It is less straightforward to draw conclusions from the absence of a significant drug effect on normal practice dynamics. This could imply that practice effects are not normally mediated by dopaminergic, cholinergic or GABAergic systems and, indeed, there is some prior data to suggest that repetition suppression is not susceptible to modulation by cholinergic manipulation with scopolamine in monkeys (Miller and Desimone, 1993; Desimone, 1996). However, there remains the possibility of false negative (type 2) error due, for example, to the modest sample size or drug doses used in this study. In this respect it is notable that although diazepam induced a marked increase in salience of practice effects in brain systems normally adaptive to practice this did not attain statistical significance as diazepam treatment was also associated with increased variability of practice effects. Furthermore, diazepam did cause significant, atypical modulation of practice effects in occipital and temporal regions adjacent to systems normally adaptive to practice. It remains conceivable therefore that adaptivity to practice may be preferentially sensitive to GABAergic manipulation, in contrast to the relatively powerful effects of cholinergic and dopaminergic manipulations on load response.
It is interesting to consider in greater detail the role of the anterior cingulate cortex (ACC), which was the only region significantly adaptive to both practice and difficulty. Activation of ACC has been seen in a wide variety of tasks with no clear specificity for language, memory or attention. For this reason, a more general role has been postulated, which would be consistent with our observation that ACC is preferentially activated both when a task is unpracticed and when it places a higher load on memory. An important question, therefore, concerns the precise nature of this ‘higher order’ role. While frequently activated in association with memory tasks, both during encoding and retrieval (Shallice et al., 1994; Rugg et al., 1996), more precise understanding of anterior cingulate function has been gained from non-mnemonic tasks, mostly relating to attention and response selection. For example, it has been suggested that ACC is important for detecting conflict when several responses are possible (Botvinick et al., 1999). In the context of a ‘flanker’ task, in which a central cue determining the subject’s response is flanked by cues that may or may not be compatible with it, anterior cingulate activation is maximal when the flanking cues are incompatible with the central cue. Similarly, it has been shown that anterior cingulate activation is conditional on conflict within a stimulus and on conflict produced by a cue configuration that violates an expectancy based on preceding trials, e.g. a compatible trial preceded by several incompatible ones or vice versa (Casey et al., 2000). An alternative formulation, based on the observation that ACC is activated equally by go and no-go trials in the context of a go/no-go motor inhibition task presented with equal frequencies of go and no-go trials, is that ACC may be generally important for ‘making and monitoring decisions’ (Liddle et al., 2001). In this study, the unpracticed and high load conditions would provoke both a high likelihood of error and also an enhanced requirement for making and monitoring decisions.
It is also worth considering the different dynamics of activation demonstrated by anterior cingulate and dorsolateral prefrontal cortex, which was also adaptive to difficulty but not to practice. Gehring and Knight showed that an electrophysiological marker of error was preserved in patients with lateral prefrontal (but not anterior cingulate) damage but the patients were unable to compensate for errors by modifying responses to subsequent trials (Gehring and Knight, 2000). There is further evidence for dissociable functions of anterior cingulate and dorsolateral prefrontal cortices in the Stroop interference task (MacDonald et al., 2000). When a subject has been alerted that they must imminently overcome interference, activation of left dorsolateral prefrontal cortex is greater than when there is no need to prepare for conflict. In contrast, anterior cingulate activation was insensitive to warnings of imminent conflict but was differentially activated at the time of task performance. These results suggest that anterior cingulate and dorsolateral prefrontal cortex may be specialized for performance monitoring and control implementation, respectively. In this study, the high load condition is likely to make greater demands on both processes, accounting for load responsivity of both cingulate and lateral prefrontal areas. With practice, the task becomes more automatic and perhaps requires less monitoring, leading to an adaptive reduction in activation of ACC.
Finally, we note that the functional anatomy of typical adaptivity to load and repetition we have defined in this study should be regarded provisionally as task specific. There is some interesting prior work to suggest that diverse parametrically designed cognitive activation experiments, e.g. demanding executive control, response selection, memory and problem-solving, all elicit load-related activation quite consistently in ventro- and dorso-lateral prefrontal cortex and in dorsal ACC (Duncan and Owen, 2000). Our findings of adaptivity to difficulty of object-location learning in anterior cingulate, middle and inferior frontal gyri are clearly consistent with this pattern of a generally load-responsive frontal system (see Table 2 for details). However, there have also been reports of difficulty-related activation of parietal cortical regions by working memory paradigms (Braver et al., 1997; Jonides et al., 1997; Honey et al., 2000), which we have not replicated here, presumably because this object-location learning task makes minimal demands on working memory. The implication is that load response in parietal cortex, perhaps because it is related specifically to variable demand for short-term phonological or visuospatial maintenance, is more conditional on cognitive task details than load response in frontal cortex. Likewise, our definition of a posterior system adaptive to task repetition apparently departs from several previous studies that have shown that middle and inferior frontal gyri may also show habituation of signal on repeated task presentation (Raichle et al., 1994; Petersen et al., 1998; Fletcher et al., 2000, 2001). Our preferred working hypothesis to explain this discrepancy is in terms of regionally variable time constants for adaptivity to repetition. Frontal adaptivity to repetition is typically item-specific and relatively rapid: for example, Fletcher et al. (Fletcher et al., 2001) reported habituation of frontal signal within the first 15 trials of an associative learning task. However, in this experiment, the response to between-block repetition was modeled over 48 trials, each block (pair of epochs) comprising 12 trials of two types identically repeated. It is therefore possible that frontal habituation might have occurred largely or completely within the first pair of epochs and so will not have been detected by our analysis focused on longer-term between-block repetition effects. This hypothesis, that frontal cortex adapts more rapidly than occipito-temporal cortex to stimulus or task repetition, could clearly be experimentally tested by future studies using event-related designs to allow a finer-grained characterization of habituation dynamics on a trial-by-trial basis.
Drug Effects on Atypical Adaptivity to Difficulty and Practice
Thus far we have considered our findings primarily with the aim of using a variety of drug challenges to distinguish separable dynamics of normal or typical neurocognitive activation. However, the effects of various drug treatments on atypical brain activation are also of interest for what they reveal about the drugs themselves. Broadly these can be divided on the basis of our results into those drugs (diazepam and methylphenidate) which atypically enhanced brain activation dynamics and those (sulpiride and scopolamine) which atypically attenuated activation dynamics compared to placebo.
Methylphenidate is a stimulant and an indirect monoaminergic agonist that increases the synaptic availability of dopamine by blocking active transport mechanisms for dopamine re-uptake; sulpiride is a relatively selective dopamine D2 receptor antagonist. In light of the opposite effects these two drugs have on dopaminergic transmission, it is interesting that they had analogous effects on neurocognitive function, and that their divergent effects on brain activation were compatible with prior work linking low dopaminergic activity to reduced frontal cortical function (Weinberger et al., 1988; Honey et al., 1999; Egan et al., 2001). Methylphenidate significantly enhanced activation–baseline contrast in right prefrontal and left ventral occipital and medial temporal lobe structures and also significantly enhanced load response in medial posterior parietal, temporoparietal, lateral premotor and anterior cingulate cortices. Sulpiride significantly attenuated load response in these regions, all of which have previously been identified as epicenters of a large-scale neurocognitive network for spatial attention (Gitelman et al., 1999; Mesulam, 2000). Modulation of this network by methylphenidate has been previously demonstrated by PET (Mehta et al., 2000). Comparable effects have also been reported by Vaidya et al. (Vaidya et al., 1998) who used a region of interest (ROI) analysis to show enhanced activation–baseline contrast in fMRI data acquired following methylphenidate in all major frontal regions. Our results more specifically suggest that recruitment of spatial attentional systems in the face of increased visuospatial load may be mediated dopaminergically such that drugs attenuating dopaminergic transmission will tend to inhibit load responsivity in this network, whereas drugs enhancing dopaminergic transmission will tend to facilitate it. If corroborated by further studies of other dopaminergic drug effects on visuospatially demanding tasks, this generalization might provide a rationale for methylphenidate administration in clinical treatment of attention deficit hyperactivity disorder, a childhood behavioral syndrome that has been associated with anatomical deficits in cortical and subcortical components of large-scale attentional networks (Overmeyer et al., 2001).
Diazepam is an agonist at the benzodiazepine receptor linked to the GABA complex; scopolamine is a selective antagonist at muscarinic M2 receptors. The differential effects of these drugs on brain activation cannot therefore be understood so straightforwardly in terms of their opposing effects on the same neurotransmitter system. Scopolamine significantly attenuated periodic activation but did not cause atypical adaptivity to task difficulty or practice. This attenuating effect of scopolamine on periodic activation of multiple regions (including occipital and inferior temporal cortex) is entirely consistent with the opposite, enhancing effect of physostigimine (an indirect cholinergic agonist) on ventral extrastriate cortical activation related to working memory (Furey et al., 2000). These results together suggest that cholinergic activity may generally enhance neurocognitive activation and that cholinergic drugs with opposing actions at cholinergic synapses can have opposing effects on large-scale brain activation analogous to the opposing effects of dopaminergic agonist and antagonist drugs discussed above. The attenuation of periodic activation caused by scopolamine in this experiment might more speculatively suggest that brain activation is normally supported by a tonic cholinergic drive that can be interrupted by muscarinic receptor blockade.
The activation enhancing effects of diazepam were marked and extensive, both anatomically and physiologically, in the sense that diazepam not only enhanced periodic activation but also induced significant adaptivity to difficulty and practice in many brain regions that were not so adaptive in placebo-treated data. These observations are compatible with the view that, by its actions at the GABA receptor, diazepam may have important disinhibitory effects on brain activation. Comparable effects were not demonstrated in the only prior fMRI study of benzodiazepines (Thiel et al., 2001), which showed attenuation of repetition suppression by lorazepam in a group of young adults with a mean age of 23 years. However, normal aging is associated with major functional changes in neurotransmitter systems (Wenk et al., 1989; Arnsten et al., 1995) which may be measurable in terms of altered pharmacological modulation of fMRI signals (Zhang et al., 2001). We suggest that disinhibition of brain function by diazepam in a group with a mean age of 72 years is compatible with the clinical paradox that typically sedative benzodiazepines can have arousing or confusing effects in elderly patients.
One of our motivations was to assess the feasibility of pharmacological fMRI for investigating drug effects on brain function in a group of elderly participants. Our findings indicate that this can be an acceptable and informative method in this age group but it would be premature to generalize too widely on the basis of these results alone. We have compared our results to the existing literature wherever possible but all of these prior studies have investigated younger samples. Some of our results, like the enhancing effects of methylphenidate on atypical activation of frontal cortex, are similar to results previously reported (Vaidya et al., 1998) in much younger participants and may be developmentally insensitive; other results, like the disinhibiting effect of diazepam noted above are apparently different to prior data on younger participants (Thiel et al., 2001) and may indicate age-related changes in the functional effects of relevant transmitter systems. An important next step will clearly be to use pharmacological fMRI more directly as a tool for assaying normal age-related changes in human neurotransmitter systems by estimating drug effects on brain function in young and older participants investigated as part of a single design.
Acknowledgments
This work was supported by an experimental medicine research grant from GlaxoSmithKline to E.T.B. and others; and by the Wellcome Trust. We gratefully thank the volunteers who participated in this study for their cooperation and colleagues at the MRI Unit, Maudsley Hospital, London UK for technical assistance.
References
- Arnsten AFT, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline – the effects of quinpirole on memory and motor performance in monkey. J Neurosci. 1995;15:3429–3439. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland A. Acetylcholine: a neurotransmitter for learning and memory? Brain Res Rev. 1996;21:285–300. doi: 10.1016/0165-0173(95)00016-x. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brammer MJ, Bullmore ET, Simmons A, Williams SCR, Grasby PM, Howard RJ, Woodruff PWR, Rabe-Hesketh S. Generic brain activation mapping in fMRI: a non-parametric approach. Magn Reson Imag. 1997;15:763–770. doi: 10.1016/s0730-725x(97)00135-5. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Büchel C, Coull JT, Friston KJ. The predictive value of changes in effective connectivity for human learning. Science. 1999;283:1538–1541. doi: 10.1126/science.283.5407.1538. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schachter DL, Rosen BR, Dale A. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Brammer MJ, Rabe-Hesketh S, Curtis VA, Morris RG, Williams SCR, Sharma T, McGuire PK. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp. 1999a;7:38–48. doi: 10.1002/(SICI)1097-0193(1999)7:1<38::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imag. 1999b;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Long CJ, Suckling J, Fadili J, Calvert GA, Zelaya FO, Carpenter TA, Brammer MJ. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp. 2001;12:61–78. doi: 10.1002/1097-0193(200102)12:2<61::AID-HBM1004>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Keller TA, Eddy W. Graded functional activation in the visuospatial system with the amount of task demand. J Cogn Neurosci. 1999;11:9–24. doi: 10.1162/089892999563210. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA. Dissociation of response conf lict, attentional selection and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci USA. 2000;97:8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci USA. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neuronal activity to the BOLD hemodynamic response. Neuroimage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val 108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. ‘Sculpting the response space’ – an account of left prefrontal activation at encoding. Neuroimage. 2000;12:404–417. doi: 10.1006/nimg.2000.0633. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Anderson JM, Shanks DR, Honey R, Carpenter TA, Donovan T, Papadakis N, Bullmore ET. Responses of human prefrontal cortex to surprising events are predicted by formal associative learning theory. Nat Neurosci. 2001;4:1043–1048. doi: 10.1038/nn733. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Zarahn E, Josephs O, Henson RNA, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Furey M, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim Y-H, Meyer JR, Mesulam M-M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FIM. The effects of age on the neural correlates of episodic encoding. Cereb Cortex. 1999;9:805–814. doi: 10.1093/cercor/9.8.805. [DOI] [PubMed] [Google Scholar]
- Grasby PM, Frith CD, Friston KJ, Simpson J, Fletcher PC, Frackowiak RSJ, Dolan RJ. A graded task approach to the functional mapping of brain areas implicated in auditory–verbal memory. Brain. 1994;117:1271–1282. doi: 10.1093/brain/117.6.1271. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Dolan RJ. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Honey GD, Bullmore ET, Soni W, Varatheesan M, Williams SCR, Sharma T. Differences in frontal cortical activation by a working memory task following substitution of risperidone for typical antipsychotic drugs in schizophrenic patients. Proc Natl Acad Sci USA. 1999;96:13432–13437. doi: 10.1073/pnas.96.23.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Bullmore ET, Sharma T. Prolonged reaction time to a verbal working memory task predicts increased power of posterior parietal cortical activation. Neuroimage. 2000;12:495–503. doi: 10.1006/nimg.2000.0624. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal working memory load affects regional brain activation as measured by PET. J Cogn Neurosci. 1997;9:462–473. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience driven changes in primary motor cortex. Proc Natl Acad Sci USA. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SB, de Ruyter van Steveninck RR, Anderson JC. The metabolic cost of neural information. Nat Neurosci. 1998;1:36–41. doi: 10.1038/236. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, Berman KF, Goldberg TE, Weinberger DR. Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage. 2000;12:268–275. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65, 1–6. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M. Principles of behavioral and cognitive neurology. Oxford University Press; New York: 2000. [Google Scholar]
- Miller EK, Desimone R. Scopolamine affects short-term memory but not inferior temporal neurons. Neuroreport. 1993;4:81–84. doi: 10.1097/00001756-199301000-00021. [DOI] [PubMed] [Google Scholar]
- Naccache L, Dehaene S. The priming method: imaging unconscious repetition priming reveals an abstract representation of number in the parietal lobes. Cereb Cortex. 2001;11:966–974. doi: 10.1093/cercor/11.10.966. [DOI] [PubMed] [Google Scholar]
- Ng VWK, Bullmore ET, de Zubicaray GI, Cooper A, Williams SCR. Identifying critical nodes in large-scale cortical networks subserving visuospatial processing: an illustration using fMRI. J Cogn Neurosci. 2001;13:537–545. doi: 10.1162/08989290152001943. [DOI] [PubMed] [Google Scholar]
- Overmeyer S, Bullmore ET, Suckling J, Simmons A, Williams SCR, Santosh PJ, Taylor E. Distributed grey and white matter deficits in hyperkinetic disorder: MRI evidence for anatomical abnormality in an attentional network. Psychol Med. 2001;31:1425–1435. doi: 10.1017/s0033291701004706. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Neural systems for perceptual skill learning. Behav Cogn Neurosci Rev. 2001;1:77–83. doi: 10.1177/1534582302001001005. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Moyano J Creso, Myers C, Gluck MA. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of cholinergic and dopaminergic afferents in the rat prefrontal cortex to learning, memory and attention. Psychobiology. 2000;28:238–247. [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, Petersen SE. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Frith CD, Frackowiak RSJ, Dolan RJ. Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Shallice T, Fletcher PC, Frith CD, Grasby PM, Frackowiak RSJ, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. A coplanar stereotactic atlas of the human brain. Thieme Verlag; Stuttgart: 1988. [Google Scholar]
- Thiel CM, Henson RNA, Morris JS, Friston KJ, Dolan RJ. Pharmacological modulation of behavioral and neuronal correlates of repetition priming. J Neurosci. 2001;21:6846–6852. doi: 10.1523/JNEUROSCI.21-17-06846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Moussai J, Zohoori S, Goldkorn A, Khan AA, Mega MS, Small GW, Cummings JL, Toga AW. Cortical variability and asymmetry in normal aging and Alzheimer’s disease. Cereb Cortex. 1998;8:492–509. doi: 10.1093/cercor/8.6.492. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JDE. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance imaging study. Proc Natl Acad Sci USA. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Illowsky BP. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia III. A new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry. 1988;45:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC. Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobiol Aging. 1989;10:11–19. doi: 10.1016/s0197-4580(89)80005-3. [DOI] [PubMed] [Google Scholar]
- Zhang ZM, Andersen A, Grondin R, Barber T, Avison R, Gerhardt G, Gash D. Pharmacological MRI mapping of age-associated changes in basal ganglia circuitry of awake rhesus monkeys. Neuroimage. 2001;14:1159–1167. doi: 10.1006/nimg.2001.0902. [DOI] [PubMed] [Google Scholar]