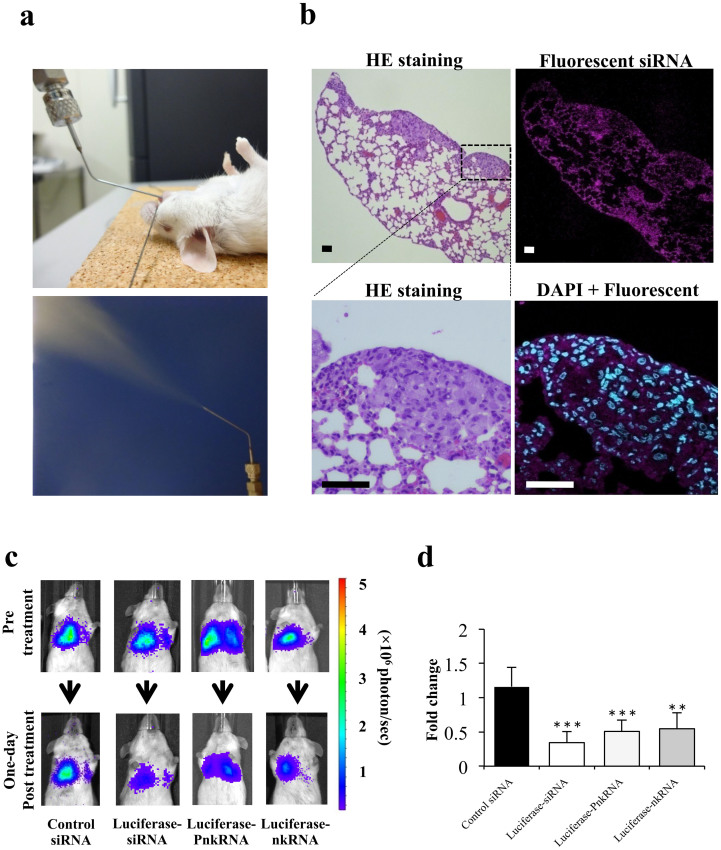
Figure 2. Inhaled single administration study with novel RNAi platforms against luciferase gene in vivo.
(a) Intratracheal delivery route: RNAi therapeutic agents are sprayed directly from the mouth into the lungs using a MicroSprayer™ aeroliser. (b) Distribution of fluorescent siRNA in the lungs after inhalation. A sufficient pulmonary distribution of aerosolised siRNA was attained in mice by MicroSprayer™. In addition, intracellular fluorescent staining in lung cancer cells and bronchial epithelial cells was occasionally observed (magnified image, DAPI + Fluorescent siRNA). The scale bars indicate 50 μm. HE, Haematoxylin-eosin. (c) Monitoring luciferase inhibition in vivo with bioluminescent imaging. Representative images pre-treatment and on the first day post-treatment. (d) Normalised fold change (one day post-treatment/pre-treatment) of bioluminescence emitted from the whole bodies of mice. The data represent the means ± SD (n = 4). Statistical analysis was performed by the Bonferroni multiple-comparison test.***, P < 0.001 versus control siRNA group. **, P < 0.01 versus control siRNA group.