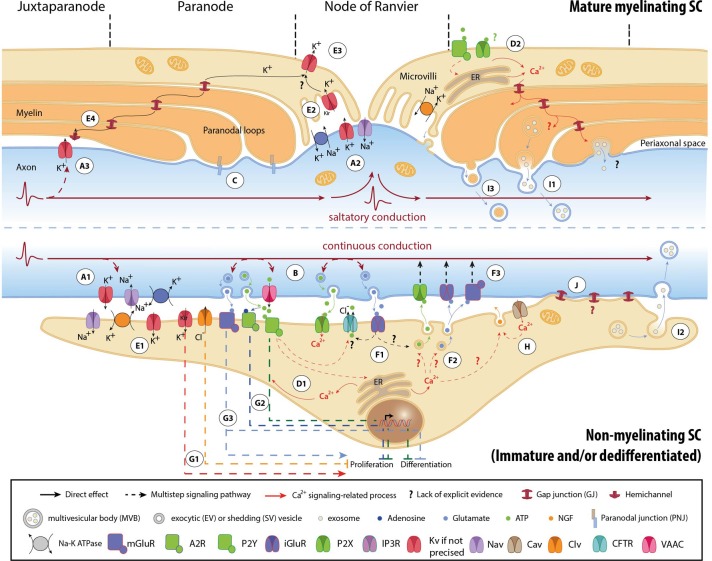
Figure 1.
Mechanisms involved in activity-dependent axon-Schwann cell bilateral communication. Schematic representation of the different molecules and mechanisms described in myelinated (upper part) and non-myelinated (lower part) PNS fibers. (A) Ephaptic communication through ion flows across the plasmalemma of unmyelinated (A1) and myelinated axons (A2, A3). (B) Paracrine signaling from axons to SCs. (C) Physical coupling between axons and mSCs. (D) SC Ca2+ transients developing after neuronal stimulation. In nmSCs activation of purinergic receptors leads to increase of cytoplasmic Ca2+ due to influx from the extracellular space, or efflux from intracellular stores (D1) (Stevens et al., 1998; Stevens and Fields, 2000; Stevens et al., 2004). mSCs express both P2X and P2Y receptors, and also respond to ATP stimulation by Ca2+ increase (D2) (Mayer et al., 1998; Grafe et al., 1999). Indications suggest that Ca2+ transients expand in the whole paranodal region through GJs (Toews et al., 2007). The origin of ATP in mature myelinated fibers, however, is not clear. High ATP levels, sufficient to activate glial receptors, are probably generated only during high frequency activity or after injury. (E) K+ buffering and ion homeostasis. K+ uptake by nmSCs through the Na+/K+ pump and KV channels (E1) (Robert and Jirounek, 1994). In mSCs, inward rectifying KV channels (IRK1/Kir2.1 and IRK3/Kir2.3), and Na+/K+ ATPases are concentrated in microvilli (E2), where massive increase of K+ occurs during neuronal activity (Mi et al., 1996; Baker, 2002). Abaxonal KV1.5 channels in the nodal area may further assist to K+ removal (E3) (Mi et al., 1995; Baker, 2002). In juxtaparanodal and internodal regions, axonal KV1 channels may act in conjunction with closely apposed SC hemichannels and with GJs of the Schmidt-Lanterman incisures (SLIs) for the same purpose (E4, see also A3) (Altevogt et al., 2002; Mierzwa et al., 2010; Nualart-Marti et al., 2013). (F) Paracrine signaling from SCs to axons. Activation of P2Y and AMPA receptors acts in a positive feedback loop, triggering ATP release by nmSCs, through vesicular exocytosis or via ion transporters, such as CFTR (F1) (Liu and Bennett, 2003; Liu et al., 2005). Administration of ATP on proliferating SCs induces secretion of the excitatory amino acids Glu and aspartate, via intracellular Ca2+ store-dependent mechanisms (F2) (Jeftinija and Jeftinija, 1998). ATP and excitatory amino acids can reciprocally bind to ionotropic and metabotropic Glu-, and P2X-receptors on unmyelinated peripheral axons and influence their excitability (F3) (Agrawal and Evans, 1986; Kinkelin et al., 2000; Carlton et al., 2001; Irnich et al., 2001). (G) Regulation of SC fate by neuronal activity through activation of ion channels (G1) (Wilson and Chiu, 1993; Pappas and Ritchie, 1998; Sobko et al., 1998), purinergic metabotropic P2Y1 receptors and A2A GPCRs by ATP and its metabolite adenosine (G2) (Stevens and Fields, 2000; Stevens et al., 2004; Fields and Burnstock, 2006), and of mGluRs (G3) (Saitoh and Araki, 2010). (H) Neurotrophic axonal support by SCs. (I) Vesicular transfer of molecules from SCs to axons. Exosomes, which are enclosed in multivesicular bodies (MVB), move from mSCs to axons through cytoplasmic-rich regions like the SLIs and paranodal domains (I1), or can be released from dedifferentiated/iSCs close to neuronal growth cones after injury (I2) (Lopez-Verrilli and Court, 2012). Shedding vesicles (SVs) are directly generated from SC plasma membrane evaginations usually in microvilli and paranodal areas of mSCs, and can fuse or be endocytosed by axons (I3) (Court et al., 2008; Cocucci et al., 2009; Lopez-Verrilli and Court, 2012). (J) Potential direct transfer route of SC molecules via GJs. Abbreviations: CaV, voltage-gated Ca2+ channel; ClV, voltage-gated Cl− channel; KV, voltage-gated K+ channel; Kir, inwardly rectifying K+ channel, NaV, voltage-gated Na+ channel; CFTR, Cystic Fibrosis Transmembrane conductance Regulator; VAAC, Volume-Activated Anion Channel; A2R, adenosine receptor 2; P2X and P2Y, purinergic receptor; iGluR, ionotropic glutamate receptor; mGluR, metabotropic glutamate receptor; GPCR, G-protein coupled receptor; NGF, nerve growth factor; ER, Endoplasmic Reticulum.