Abstract
The Drosophila annotated gene CG5155 encodes a protein that contains 10 Armadillo-repeats and has an unknown function. To fill this gap, we performed loss-of-function studies using RNAi. By analysis of four independent Drosophila RNAi lines targeting two non-overlapping regions of the CG5155 transcript, we demonstrate that this gene is required for male fertility. Therefore, we have named this gene Gudu. The transcript of Gudu is highly enriched in adult testes. Knockdown of Gudu by a ubiquitous driver leads to defects in the formation of the individualization complex that is required for spermatid maturation, thereby impairing spermatogenesis. Furthermore, testis-specific knockdown of Gudu by crossing the RNAi lines with the bam-Gal4 driver is sufficient to cause the infertility and defective spermatogenesis. Since Gudu is highly homologous to vertebrate ARMC4, also an Armadillo-repeat-containing protein enriched in testes, our results suggest that Gudu and ARMC4 is a subfamily of Armadillo-repeat containing proteins that may have an evolutionarily conserved function in spermatogenesis.
Keywords: Gudu, Drosophila, Spermatogenesis, Individualization complex, ARMC4, Armadillo repeats
1. INTRODUCTION
The study of conserved protein domains often provides important insights into the function and evolution of gene and protein families. Armadillo-repeat (ARM-repeat) motif was first identified in the Drosophila melanogaster β-catenin ortholog Armadillo (Peifer et al., 1994; Choi and Weis, 2005). Each ARM-repeat is ~42 amino acids long and the number of repeats in different proteins is highly variable, ranging from 3 to 60. Structural analysis reveals that an ARM-repeat is composed of three α-helices. The tandem ARM-repeat units fold as a superhelix, providing a platform for interaction with other protein partners (Huber et al., 1997; Choi and Weis, 2005; Kidd et al., 2005; Otomo et al., 2005; Striegl et al., 2010; Tewari et al., 2010).
Even though highly divergent ARM-repeat sequences share structural similarity, it is sometimes difficult to define cross-species orthologs and conserved biological functions based on the ARM-repeat sequences alone due to the degeneration of the sequences among the members of this protein family. Therefore, we initiated the functional study of a Drosophila ARM-repeat containing (ARMC) protein, annotated as CG5155 in the Drosophila genome. Knockdown of this gene transcript, either ubiquitously or specifically in testis, caused male sterility. Therefore we have named this ARMC protein Gudu (Chinese, meaning alone without progenies).
In animals, spermatogenesis is a conserved process that produces mature haploid sperms capable of fertilizing oocytes (Roosen-Runge, 1952). Spermatogenesis involves a series of tightly regulated stages including mitotic proliferation, meiotic division, and cellular remodeling (Sanders and Smith, 2011). The adult Drosophila testis is a bipolar tube-like structure. At the apical end of the tube, germline stem cells divide to produce spermatogonial cells, which go through mitotic and meiotic divisions to yield a group of 64 inter-connected early spermatids. The spermatids differentiate into the characteristic elongated shape and separate from each other through a process known as individualization to form mature sperms, which move into the seminal vesicle through the open basal end (Tiwari et al., 2008).
Many of the classic male sterile mutant strains show spermatid individualization failure, a defect observable by phase contrast microscopy (Fuller, 1998). Individualization initiates when the F-actin based individualization complex (IC), composed of 64 actin cones, assembles around each spermatid nucleus and moves synchronously down the length of the cyst toward the tails, expelling the excess cytosol and simultaneously wrapping each spermatid into its own plasma membrane (Fabrizio et al., 1998). Defects in IC formation and movement can lead to failure of individualization, resulting in sperm reduction and male sterility (Fabrizio et al., 1998; Sanders and Smith, 2011). Previous studies have identified many genes involved in individualization such as those coding for Auxilin, Myosin VI, Clathrin, Rab11 and Lump (Zhou et al., 2007; Sanders and Smith, 2011). These proteins either are part of the IC or regulate the integrity and membrane trafficking of the IC.
Using RNAi targeting the Gudu transcript, we show that loss of Gudu function leads to male, but not female, sterility. Moreover, Gudu RNAi flies displayed irregular IC morphology in their testes and drastically reduced number of mature sperms in their seminal vesicles. These results demonstrate that Gudu is essential for male fertility in Drosophila and suggest that Gudu plays a crucial role in spermatogenesis, particularly in the individualization process. Since ARMC4 is the closest mammalian homolog of Gudu and is also highly expressed in the mouse testes, our data suggests that ARMC4 may have a conserved function in sperm individualization and male fertility in mammalian species including humans.
2. MATERIALS AND METHODS
2.1 Fly strains and RNAi knockdown of Gudu
Information on Drosophila genes and stocks can be found in Flybase (http://flybase.org). w1118 was used as wild type controls. Gudu RNAi-1, RNAi-2, RNAi-3 (#27364, #27365, #106963) were obtained from Vienna Drosophila RNAi Center. Gudu RNAi-4 (#31568) as well as dj-GFP (#5417) was obtained from Bloomington stock center. tubulin-Gal4 was used to drive the ubiquitous expression of the double-stranded RNA (dsRNA) under the control of UAS. Testis specific bam-Gal4 was a kind gift from Dr. Xin Chen (Johns Hopkins University). Stocks were kept on cornmeal-yeast-molasses-agar media at room temperature.
2.2 Fertility assay
To examine the male fertility, five 4-day old Gudu RNAi males from each line were mated with 10 virgin w1118 females. The flies were transferred to a new vial every day, leaving the embryos on the food. The embryos laid were counted and the hatching rate was determined by dividing the number of viable moving larvae the next day by the number of embryos laid. The embryos were counted for five consecutive days, and the hatching rate was determined subsequently. For female fertility, 10 virgin Gudu RNAi females from each line were crossed with five 4-day old w1118 males. The number of embryos and hatching rate were determined as described above.
2.3 Testes phenotypic analyses
Testes of 4-day-old males were dissected out in 1XPBS buffer. For squash, dissected testes were transferred to a small drop of PBS on a microscope slide and gently pushed under the weight of the cover slip to release the testes content. The testes were observed under phase contrast settings on a Nikon Eclipse Ti microscope. To visualize the seminal vesicles and the ICs, the dissected testes were fixed in medium containing PBS and 4% formaldehyde (Mallinckrodt Chemicals) for 2 hours, and washed in PBS plus 0.1% triton-X-100. The tissues were incubated in rhodamine-conjugated phalloidin (1:500, Sigma, USA) for 1 hour at room temperature and mounted in Vectorshield with DAPI (Vector Laboratories, CA). The dj-GFP was visualized in formaldehyde fixed tissues. The testes were examined and photographed using a Nikon Eclipse Ti fluorescence microscope.
2.4 Quantitative PCR
RNA was extracted from the whole fly body or dissected testes from approximately 3 days old adult flies (control w1118 and 4 RNAi lines as listed above crossed with tubulin-Gal4 or bam-Gal4) using TRIzol Reagent (Invitrogen). Mouse tissue collection and total mRNA preparation was performed as described previously (Zhou et al., 2007). The integrity of RNA was analyzed by spectrophotometry and agarose gel electrophoresis. The RNA was treated with DNase I to remove the contaminating genomic DNA. cDNA was synthesized from 500ng total RNA using SuperScript III Reverse Transcriptase (Invitrogen). iQ SYBR Green Supermix (Bio Rad) was used to carry out the quantitative real time PCR in a C1000 Thermal Cycler (Bio Rad). rp49 expression was used as an internal control to normalize Gudu mRNA expression in flies. 5s rRNA expression was used as an internal control to normalize ARMC4 mRNA expression in mouse. The following primers were used at a concentration of 500nM: Drosophila Gudu primers 5’-CAT CGA CAT CAA GTT GAG CAT T-3’ (forward) and 5’-AAG GAG CTG AGG CAT CAA AG-3’ (reverse); Drosophila rp49 primers 5’-CGG ATG GAT ATG CTA AGC TGT-3’ (forward) and 5’-GCG CTT GTT CGA TCC GTA-3’ (reverse); mouse ARMC4 primers 5’-CAT TCC AGT GGT GGG GAC AT-3’ (forward) and 5’-ACC AGG TCT CGG GTC TCC TC-3’ (reverse); mouse 5s rRNA primers 5’-CTC CAT CCT CAT CCA ACA CC-3’ (forward) and 5’-GGA GGG GCA AGA TAC ACA GA-3’ (reverse).
3. RESULTS
3.1 CG5155 contains Armadillo-repeats and is highly enriched in adult testes
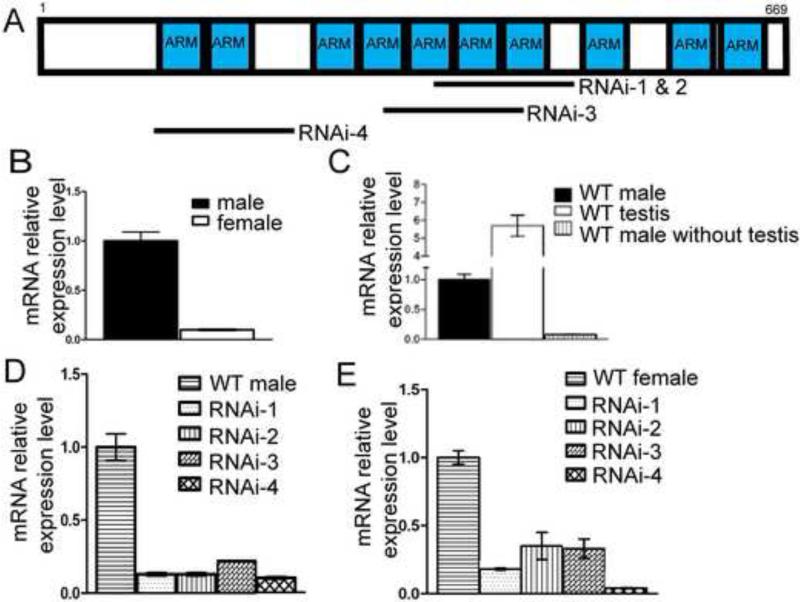
Other than the founding member β-catenin and the importins, most other ARMC proteins in the annotated Drosophila genome have not been characterized. CG5155 is a gene encoding an ARMC protein with 669 amino acids containing ten ARM-repeats (Fig. 1A) (Drysdale and Crosby, 2005) . Based on sequence similarity, this gene also has homologs in other metazoans including humans (Fig. S1). A functional study of this gene in Drosophila therefore may shed light on whether this subfamily has a conserved function. The first hint for a function of CG5155 is the observation that the transcript levels were much higher in adult male flies than females (Fig. 1B). This differential expression suggested that CG5155 might be expressed in a male specific organ. This was confirmed by a measurement of the mRNA level in testes, which showed a ~6 fold enrichment over the whole male fly (Fig.1C). Furthermore, in male carcasses that did not contain testes, the CG5155 mRNA level was ~10-fold lower compared to those with testes (Fig. 1C). Thus, CG5155 has a highly testis-enriched expression pattern.
Fig. 1.
CG5155/Gudu expression pattern and knockdown. (A) Schematic representation of CG5155/Gudu, which encodes a protein with 669 amino acids. The blue boxes indicate 10 predicted ARM repeats based on sequence similarity. The four RNAi lines targeting 3 different regions of the gene are illustrated: RNAi lines 1 and 2 are independent transgenic lines of the same construct with insertions located on chromosome 2 and 3, respectively. (B) By qPCR, male flies express ~10 times more CG5155/Gudu mRNA than the female flies. (C) The CG5155/Gudu mRNA is highly enriched in fly testis. Total mRNA was extracted from the whole male flies, dissected testes, and the carcasses excluding the testes. The CG5155/Gudu mRNA levels were measured using qPCR. A greater than 95% of CG5155/Gudu transcript in male flies is present in testes. The relative mRNA expression of CG5155/Gudu based on qPCR of RNA isolated from (D) whole male and (E) female flies. All 4 RNAi lines knocked down the expression of Gudu to ~15% or lower of the level in wild type males and to ~25% or lower of the level in wild type females. Note that the overall levels in female flies were ~10 times lower than that in male flies as shown in Fig. 1B, although the levels were set to 1 in both control flies in 1D and 1E.
3.2 Knockdown of CG5155 causes male infertility
To investigate the function of CG5155, we utilized four different transgenic strains that harbor constructs to express double stranded (ds) RNA that target three different regions of CG5155 (Fig. 1A). The expression is under the control of UAS promoter, and therefore, can be driven by Gal4 expressed in various tissues or developmental stages. While the RNAi-1, 2 and 3 lines targeted overlapping regions of CG5155 mRNA, the RNAi-4 line targeted a region that does not overlap with the target of RNAi-1, 2 or 3 (Fig. 1A). When the UAS-dsRNA strains were crossed with the ubiquitous tubulin-Gal4 driver, CG5155 mRNA levels as assayed by RT-PCR were reduced by 80-90% in male progenies of all four strains (Fig. 1D). The survival time of adult progenies carrying the driver and the dsRNA constructs was similar to that of the controls, suggesting that this knockdown or the loss of function of CG5155 did not affect the viability of the animals. Meanwhile, the lower level of expression in female flies was further reduced by ~80% after RNAi (Fig. 1E). This suggests that CG5155 is also expressed at low levels in tissues other than the testes.
Because CG5155 is highly enriched in testes, we examined whether a partial loss of CG5155 expression affected testes-related functions such as fertility. Wild type females crossed with CG5155 knockdown males laid similar number of eggs as the ones crossed with the wild type males (Table 1). However, almost all the embryos derived from CG5155 knockdown males failed to hatch (Table 1). In contrast, when the CG5155 knockdown females were crossed with wild type males, both the number of embryos laid and the hatching rate were not affected (Table 1). Therefore, CG5155 knockdown caused compromised male fertility but not female fertility. Because of the testes-enriched expression and the loss-of-function phenotype of male sterility, we have named CG5155 Gudu.
Table 1.
Infertility in Gudu knockdown males. The mRNA expression level was measured by quantitative RT-PCR using mRNA extracted from whole flies. The expression levels in wild type male or female were each set as 100% and the expression levels of the same sex flies from the RNAi lines were compared. For fertility test, 5 males from each RNAi line were crossed with 10 wild type females. The embryos laid by the 10 females were counted for the first 5 consecutive days and the percentage of hatched embryos determined on the next day. To test the female fertility, 10 Gudu knockdown females were crossed with 5 wild type males from each RNAi line and the embryos laid and hatching rate were determined.
| Gal-4 Line | Line | Gudu (CG5155) relative mRNA expression level in male | male fertility |
Gudu (CG5155) relative mRNA expression level in female | female fertility |
||
|---|---|---|---|---|---|---|---|
| No. of embryos laid by 10 mated females in 5 days | hatching rate | No. of embryos laid by 10 mated females in 5 days | hatching rate | ||||
| WT | 100%±9% | 420 | 88% | 100%±5% | 420 | 88% | |
| Tub-Gal4 | RNAi-1 | 13%±1% | 380 | 0 | 18%±0.7% | 540 | 85% |
| RNAi-2 | 13%±1% | 210 | 0 | 35%±10% | 460 | 82% | |
| RNAi-3 | 22%±0.4% | 500 | 3% | 33%±7% | 420 | 90% | |
| RNAi-4 | 10%±1% | 420 | 0 | 4%±0.3% | 450 | 86% | |
| Bam-Gal4 | RNAi-2 | 9.9%±0.8°/< | 604 | 0 | N/A | N/A | N/A |
3.3 Gudu knockdown caused abnormal sperms after meiosis
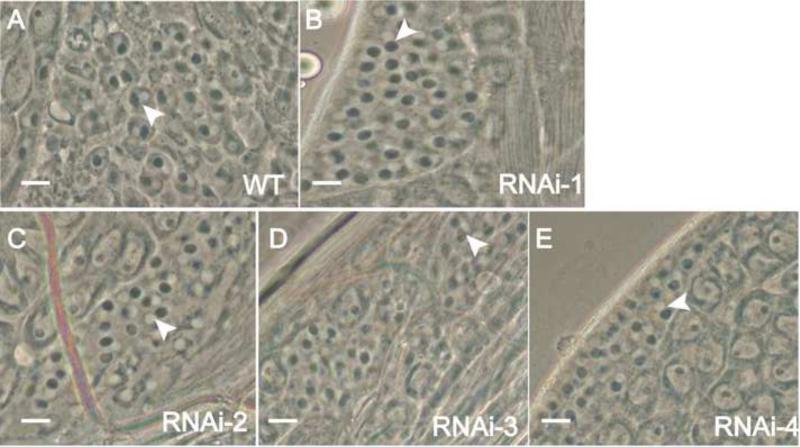
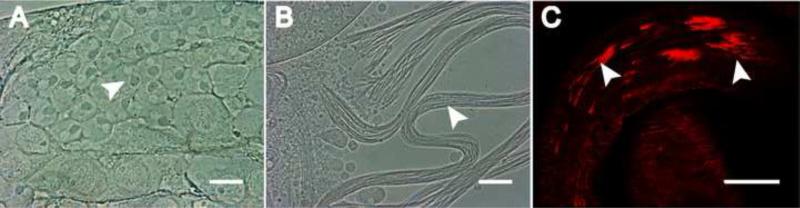
The normal number of eggs laid by the Gudu knockdown male-crossed wild type females described above suggests that the mating behavior is normal in the knockdown males. We hypothesized that the male fertility defect was caused by abnormality in one of the steps in spermatogenesis. To investigate this possibility, we examined squash preparation of testes from wild type and the RNAi males. Spermatids at onion stage in Gudu RNAi lines contained white nuclei and associated dark mitochondria nebenkern in a 1:1 ratio (Fig. 2B-E), the same as control (Fig. 2A). Furthermore, the testes in the knockdown fly contained elongated spermatids (Fig. 3), suggesting that the germline cell division, spermatogonial cell mitotic and meiotic divisions are largely normal. However, the testes in the RNAi fly contained spermatid bundles with little mobility (Fig. 3B), whereas wild type testes contained motile and individualized sperms (Fig. 3A). This kind of abnormalities was observed in all the Gudu knockdown lines.
Fig. 2.
The defect of sperm maturation was post-meiotic after Gudu RNAi. Panels A to E are phase contrast images of squashed testes from male flies with the indicated genotypes. All the wild type and RNAi lines contained normal onion-stage spermatocytes. The 1:1 ratio of the white nucleus and the associated dark mitochondria nebenkern (arrowheads) was retained in all the RNAi lines, suggesting that the spermatogenesis process is normal up to this stage. Scale bar: A-E, 10μm.
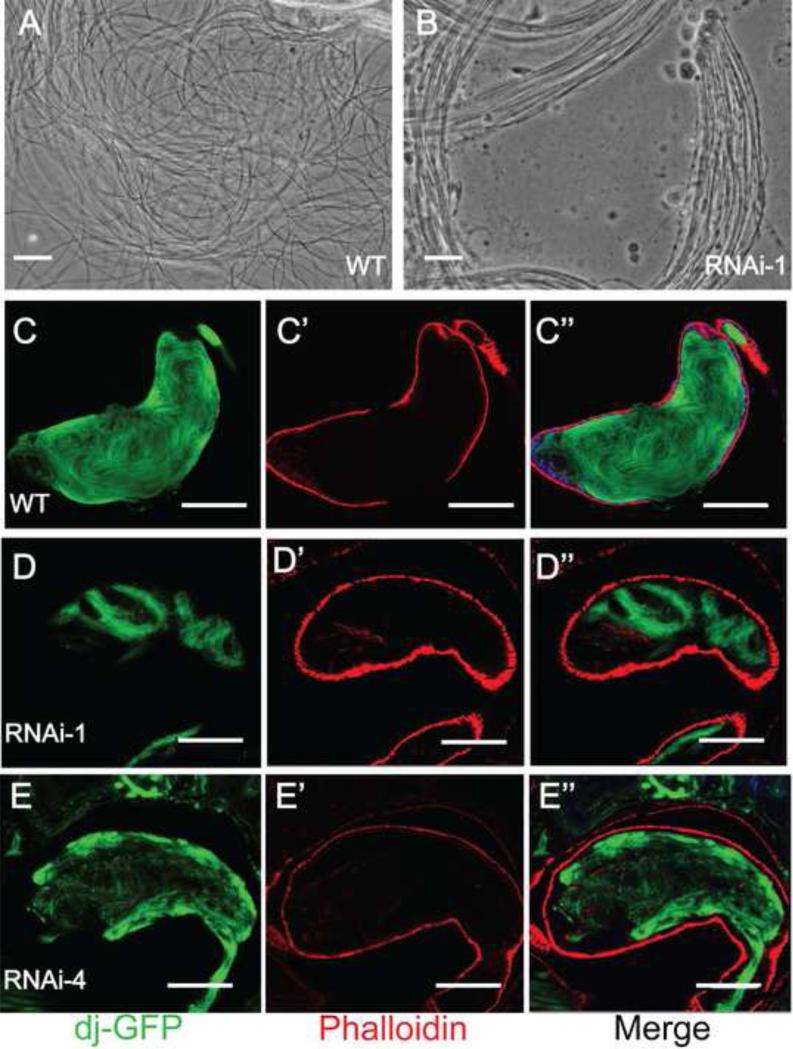
Fig. 3.
Gudu knockdown caused sperm maturation defects. Panels A and B are phase contrast images of elongated individual sperms from testes of a 2 day-old WT male (A) and bundled spermatids from a 2 day-old Gudu RNAi male (B). Note the smooth texture of individual sperm released from the squashed WT testis. In contrast, there was frequent bundling of sperms in testes of the RNAi males. Panels C to E” are DAPI and rhodamineconjugated phalloidin stained seminal vesicles of WT and Gudu RNAi lines, where the GFP tagged Don Juan protein was expressed to illustrate sperm tails. In C, abundant loose individual sperms were seen in seminal vesicle from WT male fly. In D and E, the seminal vesicles from RNAi-1 and RNAi-4 lines contained very few sperms. Scale bars in A and B, 10 µm; C-C”, 100 µm; D-E”, 50 μm. Green is dj-GFP, red is phalloidin, and blue is DAPI.
The protein Don Juan is expressed in sperm tails. Thus the line dj-GFP, which was generated by placing GFP under control of the Don Juan promoter, can be used to illuminate the late elongation-stage spermatids (Santel et al., 1997). We crossed dj-GFP with flies containing both Gudu RNAi construct and tublin-Gal4, or with wild type flies as control, and then examined whether sperms were successfully made and stored in seminal vesicles. While wild type seminal vesicles showed abundant mature individual sperms (Fig. 3C-C”), seminal vesicles from Gudu RNAi lines only contained few GFP-illuminated sperms and conspicuous bundled spermatids (Fig. 3D-E”). These results suggest that knockdown of Gudu expression leads to a post-meiotic defect, perhaps at a differentiation stage prior to sperm maturation and movement to seminal vesicles.
3.4 Knockdown of Gudu results in defective individualization complexes in testes
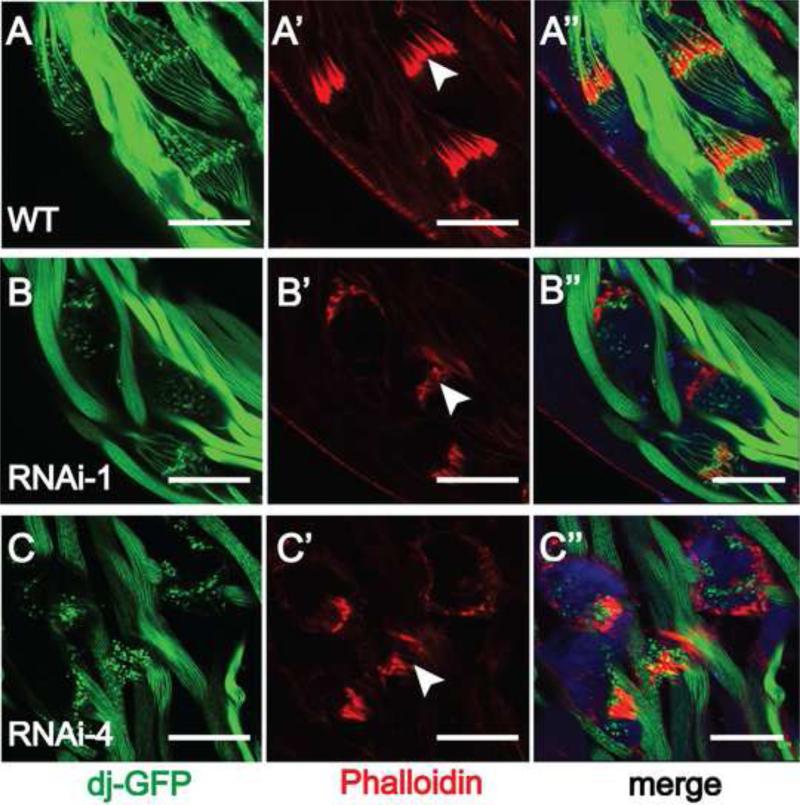
An important step of sperm maturation is the individualization of the bundled spermatids. This process is controlled initially by the IC that contains actin cones surrounding the spermatid heads. Therefore, we visualized the ICs by staining the testes with fluorescent phalloidin that binds actin. Different from the well-organized morphology seen in wild type testes (Fig. 4A’, arrow head), the ICs in the Gudu RNAi testes (Fig. 4B’ and C’, arrow heads) stained poorly, and were more disorganized and diffused. In addition, the spermatids around IC (illuminated by Don Juan-GFP) were poorly visualized (Fig. 4B” and C”), in sharp contrast with the clear spindle structure in wild type (Fig. 4A). DAPI staining for spermatid head DNA was detectable around the IC (Fig. 4C”), suggesting that the IC around the spermatid heads can be initiated but the defect develops around the time of IC formation and movement down the spermatid tail. These results suggest that loss of Gudu function leads to defects in spermatid individualization, which in turn reduces normal sperm production and causes low fertility in the male flies.
Fig. 4.
Knockdown of Gudu disrupts the individualization complex (IC). Rhodamineconjugated phalloidin stained actin, the major component of ICs. DAPI was used to stain the nuclear DNA. GFP tagged Don Juan protein (dj-GFP) illuminated spermatids. Wild type testes show highly organized ICs as a result of coordinated movement of actin cones. All 64 actin cones in each bundle are tightly located tightly together (A’, arrow head). Knockdown of Gudu led to diffused and disorganized ICs (B’ and C’, arrow head). The organized ICs were missing or only very few left in the RNAi lines. Scale bar: 40 μm. Green is dj-GFP, red is phalloidin, and blue is DAPI.
3.5 Specific knockdown of Gudu in testes causes infertility in male flies
The male infertility resulted from the ubiquitous Gudu knockdown left the possibility that the defects might be caused in part or entirely by problems in non-testes tissues. To counter this argument, we used bam-Gal4 driver line, which has been shown to drive ectopic protein expression in early-mid spermatocytes and sometimes in late-elongation stage spermatids (White-Cooper, 2012). Crossing bam-Gal4 with RNAi-2 line successfully knocked down Gudu to ~10% of the wild type mRNA level (Table 1). Consistent with the ubiquitous knockdown results, testes-specific knockdown of Gudu also led to male sterility (Table 1). Furthermore, analysis of bam-Gal4;GuduRNAi-2 male testes confirmed that the spermatids were bundled rather than individualized (Fig. 5B, arrowhead) and the ICs were disorganized (Fig. 5C, arrow head), while the 1:1 ratio of white nuclei and associated dark mitochondria nebenkern during onion stage was maintained (Fig. 5A, arrow head). These results confirm the hypothesis that the function of Gudu is critical to spermatid individualization and a loss of Gudu function in testes is sufficient to cause male sterility.
Fig. 5.
Abnormality in the testes of the male flies generated from crossing bam-Gal4 males with Gudu RNAi-2 females. A and B are phase contrast images of testes squash showing testis-specific knockdown of Gudu causes individualization defects in spermatids (B, arrowhead). The defects appeared after meiosis, because the characteristic 1:1 ratio of white nucleus and associated dark mitochondria nebenkern is retained (A, arrowhead). Rhodamin-conjugated phalloidin staining of F-actin revealed the disorganized ICs (C, arrowhead). Scale bar: A and B, 10 μm; C, 40 μm.
3.6 Gudu homolog ARMC4 is enriched in mammalian testes
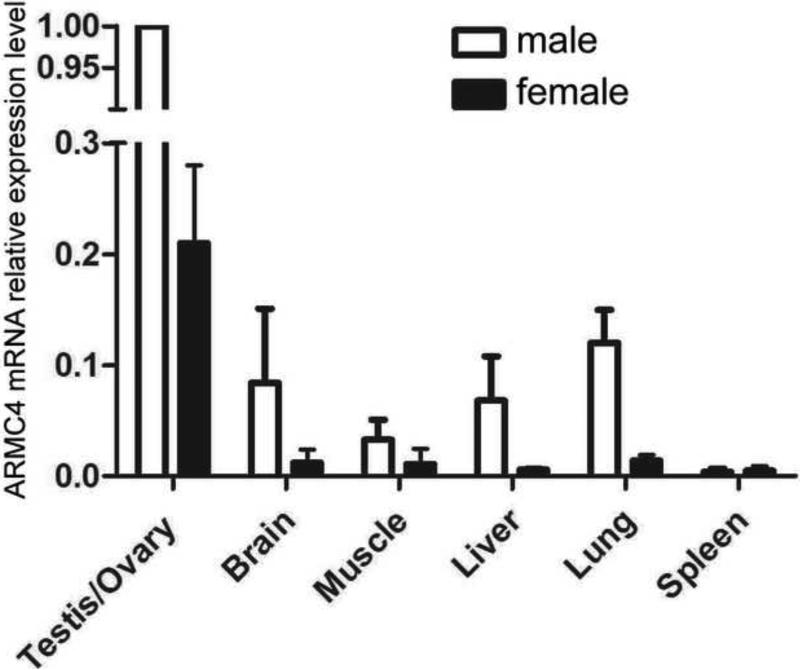
As shown in Fig. S1, Gudu is highly homologous to vertebrate ARMC4 protein family. To explore whether AMRC4 plays similar functions, we measured the mRNA levels of ARMC4 in various mouse tissues. We found that ARMC4 transcript was also highly enriched in mouse testes compared to the other tissues (Fig. 6A). This result suggests that ARMC4 may perform a similar function in mammalian spermatogenesis as Gudu in flies. Further experiments will be necessary to confirm this function in mammals.
Fig. 6.
ARMC4 is highly enriched in mouse testes. ARMC4 mRNA level in various mouse tissues was determined by quantitative PCR using 5S rRNA as internal control. The ARMC4 transcript is highly enriched in mouse testes, with very little expression in other tissues, similar to the pattern of Gudu in flies.
4. DISCUSSION
Our experiments have unveiled an essential function of Gudu/CG5155, a predicted ARMC protein in Drosophila, in male fertility. Gudu expression is highly enriched in Drosophila testes. Reduced expression of Gudu in four RNAi lines caused male infertility, but did not affect female fertility. Additionally, testis-specific knockdown of Gudu leads to the same infertility in males. By phase contrast and fluorescence microscopy, we observed a normal one-to-one nuclei-to-mitochondria nebenkern ratio at the onion stage in the Gudu knockdown flies, suggesting that the spermatogenesis went normally up to the stage of meiosis. On the other hand, we observed conspicuous abnormalities in the ICs, which strongly suggest that the individualization process is disrupted. Such a disruption is expected to impair spermatogenesis and result in few normal sperms. Indeed, we observed much reduced mature individual sperms in the seminal vesicles of the Gudu knockdown flies. These observations demonstrate that Gudu function is required in spermatogenesis, and furthermore, suggest that Gudu plays a critical role in the individualization process during spermatogenesis.
Individualization has been extensively studied in Drosophila, and many genes are involved in this process, including lump, myosinVI, auxilin and npc1 (Hicks et al., 1999; Zhou et al., 2007; Sanders and Smith, 2011; Wang et al., 2011). From these studies, actin assembly, apoptosis, and membrane trafficking become the three best understood processes shown to affect different aspects of sperm maturation. Because the IC is made of 64 actin cones, any defect in the IC formation, actin dynamics, and maintenance of IC integrity could lead to individualization failure and ultimately male infertility (Noguchi and Miller, 2003; Noguchi et al., 2006; Noguchi et al., 2008; Noguchi et al., 2009). Because ARMC proteins likely function as scaffolds for multi-protein complexes (Tewari et al., 2010), Gudu may function as an interacting partner for some of the proteins involved in the individualization process.
System analysis revealed that almost half of the genes in the Drosophila genome are expressed in testis, approximately 25% are testis-enriched and 10% (~1500 genes) contribute to male fertility (Andrews et al., 2000; Hackstein et al., 2000; Parisi et al., 2004; Chintapalli et al., 2007). Our work demonstrates that Gudu expression is highly enriched in testis and functions in male fertility. Gudu mRNA has a 10-fold lower expression in females, and knocking down Gudu by a ubiquitous driver did not affect female fertility. Furthermore, the four RNAi lines we used brought down Gudu mRNA to ~10% of the wild type level, yet the flies were viable and showed no detectable mating or behavioral defects. These results suggest that Gudu mainly functions in testis and is indispensable for spermatogenesis.
These findings raise interesting implications for the function of Gudu's mammalian homolog AMRC4. The shared sequence homology (Fig. S1) and testis-enriched expression pattern between Gudu and AMRC4 (Fig. 1 and 6) suggest that ARMC4 may play a similar role in mammalian testes. Worldwide, male infertility claims an estimated 5% victims in human male population and defects in sperm production is a common cause for this problem (Cram et al., 2004). Therefore, further studies on the role of Gudu and ARMC4 in spermatogenesis will help to understand male infertility.
Supplementary Material
Fig. S1 The closest homolog of Gudu based on sequence similarity is ARMC4 (http://pfam.sanger.ac.uk). These ARMC4 homologs in human, mouse, chicken, frog and fly constitute a subfamily of ARM-repeat-containing proteins. Sequence alignment of this subfamily was done using the online software at http://www.ibi.vu.nl/programs/pralinewww/. ARMC4 is highly conserved in vertebrates. The tandem ARM repeats are in the C-terminus. The similarities between human and mouse, human and chicken, and human and frog ARMC4 are 91%, 77% and 79%, respectively. While the vertebrate proteins are approximately 1110 a.a., Gudu is only 669 a.a. long, missing the N-terminal counterpart of vertebrate. Nonetheless, the whole Gudu protein sequence matches the a.a. 434 to the C-terminus of vertebrate ARMC4. Both vertebrate ARMC4 and fly Gudu contain 10 ARM repeats.
Highlights.
The Drosophila Armadillo repeat protein Gudu has testis-enriched expression.
Loss of function of Gudu leads to Drosophila male sterility.
Gudu acts in the testis for spermatogenesis.
Gudu is required for the proper function of spermatid individualization complex.
Acknowledgment
We are grateful to Dr. Xin Chen (Johns Hopkins University) for providing the bam-Gal4 lines and Drs. John Landers, Daryl Bosco and Fen-Biao Gao for discussion and helpful suggestions. This work was supported by a grant (5R01NS059708) from National Institute of Neurological Disorders and Stroke to ZX. YTI is supported by an NIH grant (DK83450), is a member of the UMass DERC (DK32520), a member of the UMass CCTS (UL1TR000161) and a member of the Guangdong Innovative Research Team Program (No. 201001Y0104789252).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Andrews J, Bouffard GG, Cheadle C, Lu J, Becker KG, Oliver B. Gene discovery using computational and microarray analysis of transcription in the Drosophila melanogaster testis. Genome Res. 2000;10:2030–43. doi: 10.1101/gr.10.12.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–20. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Weis WI. Structure of the armadillo repeat domain of plakophilin 1. J Mol Biol. 2005;346:367–76. doi: 10.1016/j.jmb.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Cram D, Lynch M, O'Bryan MK, Salvado C, McLachlan RI, de Kretser DM. Genetic screening of infertile men. Reprod Fertil Dev. 2004;16:573–80. doi: 10.10371/RD03097. [DOI] [PubMed] [Google Scholar]
- Drysdale RA, Crosby MA. FlyBase: genes and gene models. Nucleic Acids Res. 2005;33:D390–5. doi: 10.1093/nar/gki046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio JJ, Hime G, Lemmon SK, Bazinet C. Genetic dissection of sperm individualization in Drosophila melanogaster. Development. 1998;125:1833–43. doi: 10.1242/dev.125.10.1833. [DOI] [PubMed] [Google Scholar]
- Fuller MT. Genetic control of cell proliferation and differentiation in Drosophila spermatogenesis. Semin Cell Dev Biol. 1998;9:433–44. doi: 10.1006/scdb.1998.0227. [DOI] [PubMed] [Google Scholar]
- Hackstein JH, Hochstenbach R, Pearson PL. Towards an understanding of the genetics of human male infertility: lessons from flies. Trends Genet. 2000;16:565–72. doi: 10.1016/s0168-9525(00)02140-5. [DOI] [PubMed] [Google Scholar]
- Hicks JL, Deng WM, Rogat AD, Miller KG, Bownes M. Class VI unconventional myosin is required for spermatogenesis in Drosophila. Mol Biol Cell. 1999;10:4341–53. doi: 10.1091/mbc.10.12.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AH, Nelson WJ, Weis WI. Three-dimensional structure of the armadillo repeat region of beta-catenin. Cell. 1997;90:871–82. doi: 10.1016/s0092-8674(00)80352-9. [DOI] [PubMed] [Google Scholar]
- Kidd AR, 3rd, Miskowski JA, Siegfried KR, Sawa H, Kimble J. A beta-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell. 2005;121:761–72. doi: 10.1016/j.cell.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Frank DJ, Isaji M, Miller KG. Coiled-coil-mediated dimerization is not required for myosin VI to stabilize actin during spermatid individualization in Drosophila melanogaster. Mol Biol Cell. 2009;20:358–67. doi: 10.1091/mbc.E08-07-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Lenartowska M, Miller KG. Myosin VI stabilizes an actin network during Drosophila spermatid individualization. Mol Biol Cell. 2006;17:2559–71. doi: 10.1091/mbc.E06-01-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Lenartowska M, Rogat AD, Frank DJ, Miller KG. Proper cellular reorganization during Drosophila spermatid individualization depends on actin structures composed of two domains, bundles and meshwork, that are differentially regulated and have different functions. Mol Biol Cell. 2008;19:2363–72. doi: 10.1091/mbc.E07-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Miller KG. A role for actin dynamics in individualization during spermatogenesis in Drosophila melanogaster. Development. 2003;130:1805–16. doi: 10.1242/dev.00406. [DOI] [PubMed] [Google Scholar]
- Otomo T, Otomo C, Tomchick DR, Machius M, Rosen MK. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol Cell. 2005;18:273–81. doi: 10.1016/j.molcel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Parisi M, Nuttall R, Edwards P, Minor J, Naiman D, Lu J, Doctolero M, Vainer M, Chan C, Malley J, Eastman S, Oliver B. A survey of ovary-, testis-, and soma-biased gene expression in Drosophila melanogaster adults. Genome Biol. 2004;5:R40. doi: 10.1186/gb-2004-5-6-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Berg S, Reynolds AB. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994;76:789–91. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Roosen-Runge EC. Kinetics of spermatogenesis in mammals. Ann N Y Acad Sci. 1952;55:574–84. doi: 10.1111/j.1749-6632.1952.tb26577.x. [DOI] [PubMed] [Google Scholar]
- Sanders C, Smith DP. LUMP is a putative double-stranded RNA binding protein required for male fertility in Drosophila melanogaster. PLoS One. 2011;6:e24151. doi: 10.1371/journal.pone.0024151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striegl H, Andrade-Navarro MA, Heinemann U. Armadillo motifs involved in vesicular transport. PLoS One. 2010;5:e8991. doi: 10.1371/journal.pone.0008991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari R, Bailes E, Bunting KA, Coates JC. Armadillo-repeat protein functions: questions for little creatures. Trends Cell Biol. 2010;20:470–81. doi: 10.1016/j.tcb.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Tiwari AK, Alone DP, Roy JK. Rab11 is essential for fertility in Drosophila. Cell Biol Int. 2008;32:1158–68. doi: 10.1016/j.cellbi.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Wang C, Ma Z, Scott MP, Huang X. The cholesterol trafficking protein NPC1 is required for Drosophila spermatogenesis. Dev Biol. 2011;351:146–55. doi: 10.1016/j.ydbio.2010.12.042. [DOI] [PubMed] [Google Scholar]
- White-Cooper H. Tissue, cell type and stage-specific ectopic gene expression and RNAi induction in the Drosophila testis. Spermatogenesis. 2012;2:11–22. doi: 10.4161/spmg.19088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Falkenburger BH, Schulz JB, Tieu K, Xu Z, Xia XG. Silencing of the Pink1 gene expression by conditional RNAi does not induce dopaminergic neuron death in mice. Int J Biol Sci. 2007;3:242–50. doi: 10.7150/ijbs.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 The closest homolog of Gudu based on sequence similarity is ARMC4 (http://pfam.sanger.ac.uk). These ARMC4 homologs in human, mouse, chicken, frog and fly constitute a subfamily of ARM-repeat-containing proteins. Sequence alignment of this subfamily was done using the online software at http://www.ibi.vu.nl/programs/pralinewww/. ARMC4 is highly conserved in vertebrates. The tandem ARM repeats are in the C-terminus. The similarities between human and mouse, human and chicken, and human and frog ARMC4 are 91%, 77% and 79%, respectively. While the vertebrate proteins are approximately 1110 a.a., Gudu is only 669 a.a. long, missing the N-terminal counterpart of vertebrate. Nonetheless, the whole Gudu protein sequence matches the a.a. 434 to the C-terminus of vertebrate ARMC4. Both vertebrate ARMC4 and fly Gudu contain 10 ARM repeats.