Abstract
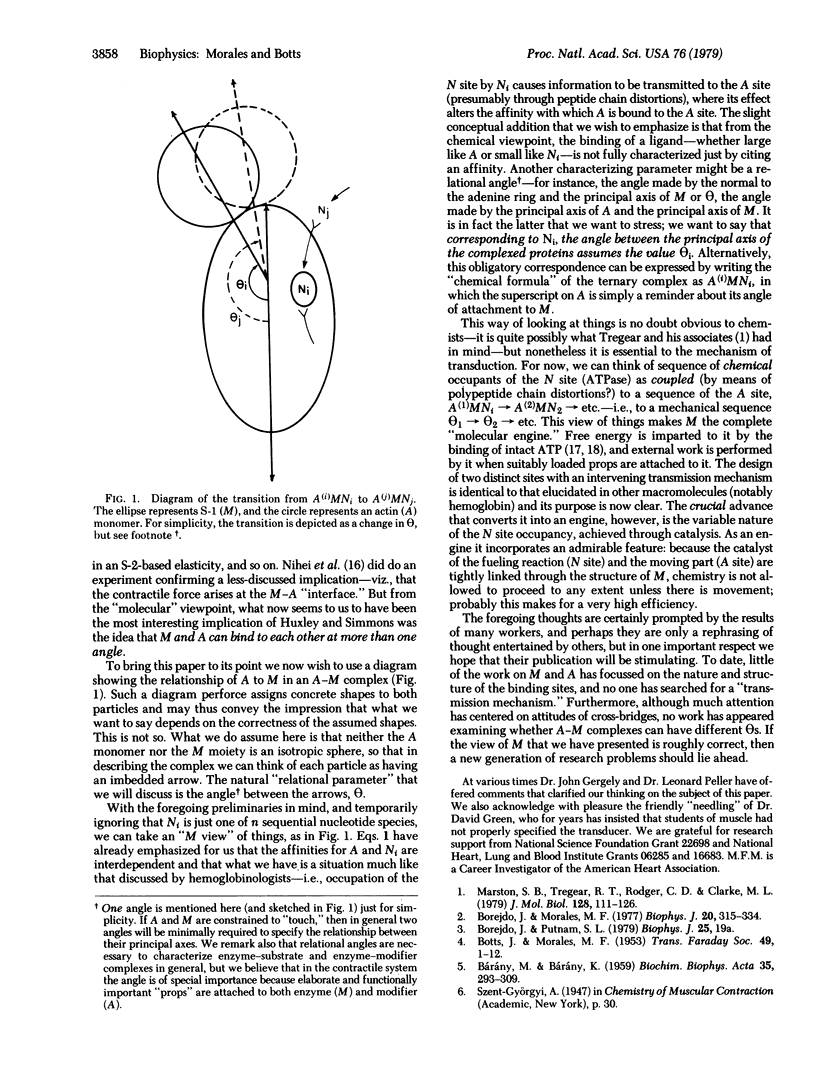
Herein it is developed that energy transduction in muscle is an activity of myosin S-1 and its ligands, actin (A) and nucleotide (N). S-1 shares with other molecular particles (e.g., hemoglobin) the property that binding events at one of its sites, the N-site, influences binding events at a remote site, the A site (specifically, influences both the actin affinity and actin attachment angle at the A site). However, there is a crucial difference between S-1 and the better-known systems. Because the N site is enzymatic, it has a temporal sequence of occupants; this imposes a temporal sequence of actin attitudes--i.e., a sequence of mechanical events.
Full text
PDF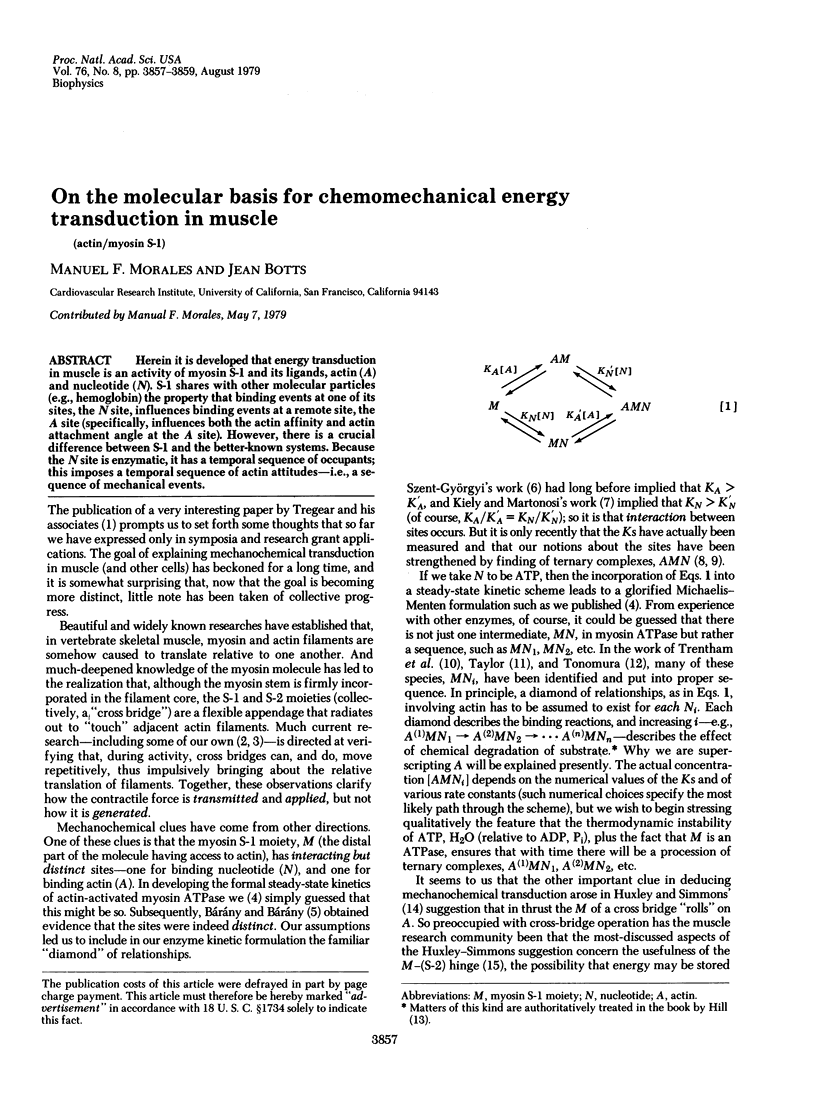

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARANY M., BARANY K. Studies on "active centers" of L-myosin. Biochim Biophys Acta. 1959 Oct;35:293–309. doi: 10.1016/0006-3002(59)90378-6. [DOI] [PubMed] [Google Scholar]
- Borejdo J., Morales M. F. Fluctuations in tension during contraction of single muscle fibers. Biophys J. 1977 Dec;20(3):315–334. doi: 10.1016/S0006-3495(77)85552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith S. Interactions of the actin and nucleotide binding sites on myosin subfragment 1. J Biol Chem. 1976 Oct 25;251(20):6170–6172. [PubMed] [Google Scholar]
- Highsmith S., Kretzschmar K. M., O'Konski C. T., Morales M. F. Flexibility of myosin rod, light meromyosin, and myosin subfragment-2 in solution. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4986–4990. doi: 10.1073/pnas.74.11.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Kiely B., Martonosi A. The binding of ADP to myosin. Biochim Biophys Acta. 1969 Jan 14;172(1):158–170. doi: 10.1016/0005-2728(69)90101-7. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Tregear R. T., Rodger C. D., Clarke M. L. Coupling between the enzymatic site of myosin and the mechanical output of muscle. J Mol Biol. 1979 Feb 25;128(2):111–126. doi: 10.1016/0022-2836(79)90121-9. [DOI] [PubMed] [Google Scholar]
- Martonosi A. The binding of Mn2+ and ADP to myosin. J Supramol Struct. 1975;3(4):323–332. doi: 10.1002/jss.400030403. [DOI] [PubMed] [Google Scholar]
- Nihel T., Mendelson R. A., Botts J. The site of force generation in muscle contraction as deduced from fluorescence polarization studies. Proc Natl Acad Sci U S A. 1974 Feb;71(2):274–277. doi: 10.1073/pnas.71.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. W. Chemistry of muscle contraction. Annu Rev Biochem. 1972;41(10):577–616. doi: 10.1146/annurev.bi.41.070172.003045. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Eccleston J. F., Bagshaw C. R. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976 May;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]


