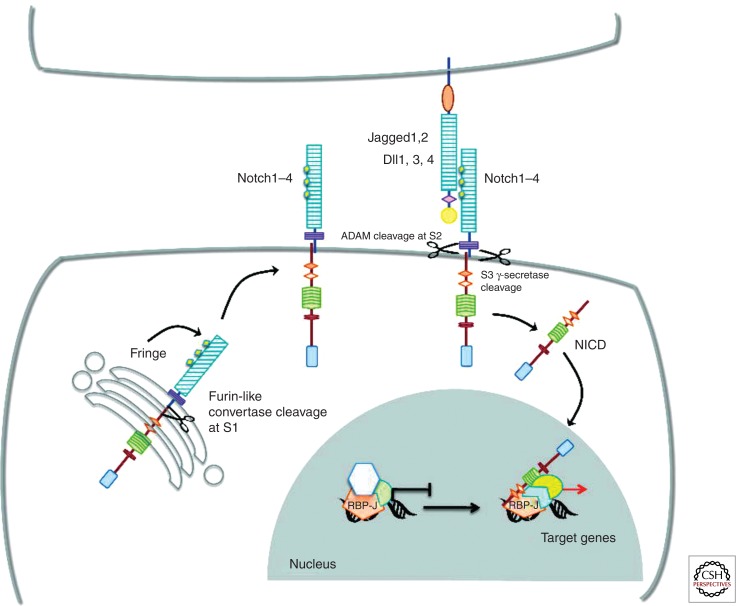
Figure 1.
Notch signaling. Vertebrates have four Notch receptors (Notch1–Notch4, N1–N4) and five conventional ligands (Jagged1, Jaggged2, Delta-like1 [Dll1], Delta-like3 [Dll3], and Delta-like4 [Dll4]). Notch proteins are synthesized as single precursor proteins, which are cleaved in the Golgi by a Furin-like convertase at site S1. Cleavage at S1 generates two subunits held together noncovalently. EGF-like repeats present within the extracellular domain of the receptors are glycosylated by Fringe proteins in the Golgi before receptors are transported to the cell surface. Notch signaling is initiated by ligand–receptor interaction, which induces a second cleavage at site S2 (close to the transmembrane domain) mediated by ADAM-type metalloproteases, followed by a third cleavage at S3 within the transmembrane domain mediated by the γ-secretase activity of a multiprotein complex containing presenilins. This last proteolytic cleavage liberates the cytoplasmic domain of Notch receptors (NICD), which translocate to the nucleus and bind to the transcription factor RBP-J, converting it from a transcriptional repressor into a transcriptional activator by recruiting coactivators to induce target gene expression.