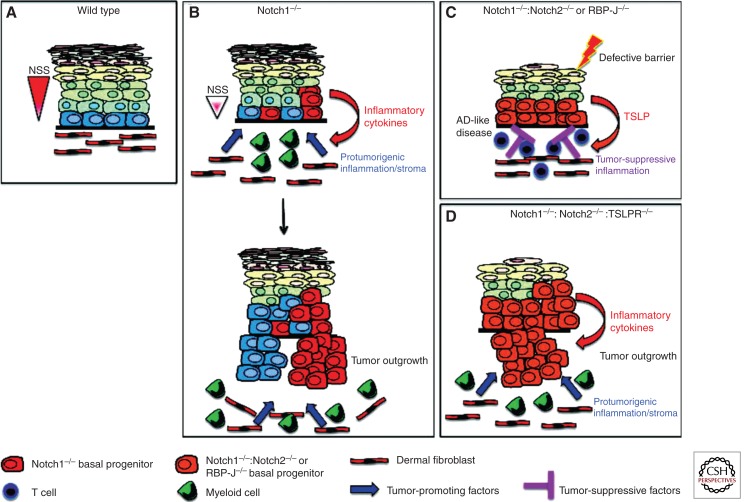
Figure 4.
A model of how aberrant Notch signaling in the IFE influences carcinogenesis by regulating inflammation. (A) Depiction of wild-type epidermis in which normal levels of Notch signaling (red triangle to the left of diagram) ensure the correct balance between proliferation and differentiation. Fibroblasts constitute a significant proportion of the underlying dermis and provide support for epidermal stem/progenitor cells. (B) Partial reduction of Notch signal strength by ablation of Notch1 results in perturbed epidermal differentiation and the secretion of proinflammatory cytokines. This leads to the infiltration of hematopoietic cells, including cells of the myeloid lineage, into the underlying dermis and the formation of a protumorigenic stroma, which promotes tumor development from Notch1−/− and wild-type epidermal cells. (C) Complete ablation of epidermal Notch signaling, by either compound deletion of Notch1 and Notch2 or ablation of RBP-J, results in extremely high levels of TSLP secretion and the subsequent development of an atopic-dermatitis (AD)-like disease. Elevated TSLP expression may occur by direct regulation of TSLP by Notch or may be a consequence of loss of barrier function. In addition to inducing AD, high levels of TSLP prevent tumor development from the IFE and its appendages by inducing T-cell-mediated antitumor immunity. (D) Abrogation of TSLP-mediated immune responses by genetic ablation of the TSLPR results in rapid tumor development from cutaneous epithelial cells. Tumor growth remains dependent on protumorigenic inflammation, which is induced by the Notch-deficient IFE. NSS, Notch signal strength.